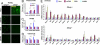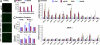Antioxidants reduce endoplasmic reticulum stress and improve protein secretion - PubMed (original) (raw)
Antioxidants reduce endoplasmic reticulum stress and improve protein secretion
Jyoti D Malhotra et al. Proc Natl Acad Sci U S A. 2008.
Abstract
Protein misfolding in the endoplasmic reticulum (ER) contributes to the pathogenesis of many diseases. Although oxidative stress can disrupt protein folding, how protein misfolding and oxidative stress impact each other has not been explored. We have analyzed expression of coagulation factor VIII (FVIII), the protein deficient in hemophilia A, to elucidate the relationship between protein misfolding and oxidative stress. Newly synthesized FVIII misfolds in the ER lumen, activates the unfolded protein response (UPR), causes oxidative stress, and induces apoptosis in vitro and in vivo in mice. Strikingly, antioxidant treatment reduces UPR activation, oxidative stress, and apoptosis, and increases FVIII secretion in vitro and in vivo. The findings indicate that reactive oxygen species are a signal generated by misfolded protein in the ER that cause UPR activation and cell death. Genetic or chemical intervention to reduce reactive oxygen species improves protein folding and cell survival and may provide an avenue to treat and/or prevent diseases of protein misfolding.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
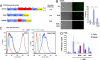
Fig. 1.
Induction of FVIII expression causes oxidative stress and apoptosis in CHO cells. (A) Schematic shows the domain structure of FVIII and deletion constructs used in these experiments. Positions of disulfide bonds and N-linked glycosylation sites are depicted. (B) wtFVIII-expressing CHO cells (CHO-FVIII) were treated with NaB (5 mM) for 24 h and then analyzed by TUNEL assay. TUNEL-positive cells were quantified from three separate experiments. (C) Control CHO cells and CHO-FVIII cells were treated with NaB for 24 h before staining with DCF for analysis by flow cytometry. Where indicated, cells were treated with NaB in the presence of BHA (10 μM). CHO cells were treated with H2O2 for 30 min before DCF staining as a positive control. (D) CHO-FVIII cells were treated with NaB in the presence or absence of BHA, ascorbic acid (500 μM; AA), or N-acetylcysteine (500 μM; NAC). Anti-oxidants were added 1 h before NaB treatment. After 24 h, conditioned medium and cells were harvested for analysis FVIII antigen. Data represent the mean of three independent experiments.
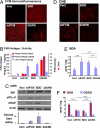
Fig. 2.
FVIII expression induces ER stress, oxidative stress, and apoptosis upon in vivo expression in liver. DNA expression vectors were delivered by tail-vein injection into WT C57BL/6 mice. After 24 h, blood and liver tissues were isolated for analysis. (A) Liver tissue sections were analyzed for immunolocalization of FVIII antigen. (B) FVIII antigen in plasma samples and liver extracts was measured by ELISA. FVIII activity in plasma samples was measured using the COAMATIC assay kit. For activity measurements, the background of murine FVIII activity was subtracted (0.35 U/ml). (C) Western blot analysis of liver tissue for detection of BiP, phospho-eIF2α, and CHOP. Densitometry indicated that BiP was increased three fold and eIF2α-P was increased two fold in mice injected with wtFVIII and BDD compared with 226/N6. Spliced Xbp1 mRNA in liver tissue was measured by real-time RT-PCR. (D) Fresh frozen liver sections were prepared and stained with 2 μM dihydroethidine hydrochloride for 30 min at 37 °C. Sections were analyzed by fluorescence microscopy. (E) Malondialdehyde was measured in liver homogenates. (F) Liver lysates were analyzed for GSH and GSH and oxidized glutathione content. Data represent the mean and SD from three different animals in B, E, and F.
Fig. 3.
Chop deletion attenuates the UPR, apoptosis, and oxidative damage upon wtFVIII and BDD expression. Chop+/+ _and Chop_−/− mice were injected with empty vector or vector containing wtFVIII, BDD, or 226/N6. Liver tissue and plasma samples were isolated after 24 h for analysis. (A) Representative images from TUNEL staining of liver sections are shown. (B) Blood was collected by retro-orbital bleed and analyzed by anti-human FVIII ELISA (n = 3). (C and D) Protein oxidation (i.e., carbonyls) and lipid peroxidation (i.e., HODEs) in liver extracts were measured as described in
SI Materials and Methods
. (E) Total RNA was isolated from livers of Chop+/+ and _Chop_−/− mice injected with empty vector, wtFVIII, BDD, or 226/N6 and analyzed by quantitative real-time RT-PCR using specific primers (
Table S1
). Values represent the mean of three mice injected with each DNA vector. The values were normalized to 18S rRNA and expressed as induction in fold relative to empty vector.
Fig. 4.
BHA feeding suppresses oxidative stress and apoptosis and improves wtFVIII and BDD secretion in vivo. WT (A-C and E) or hemophilia A _Fviii_−/− (D) mice were fed with normal chow or chow supplemented with BHA for 4 days and then DNA expression vectors were injected into the tail vein. After 24 h, plasma and liver samples were harvested: (A) TUNEL, (B) glutathione, (C) HODEs and carbonyls, and (D) real-time RT-PCR. Expression values were normalized to 18S rRNA and the fold induction is expressed relative to empty vector. B-D depict three independent mice.
Fig. 5.
BHA feeding improves secretion of wtFVIII and BDD in vivo. Hemophilia A _Fviii_−/− mice were fed with normal chow or chow supplemented with BHA for 4 days before gene delivery. (A) FVIII antigen in plasma and liver samples was measured at 24 h after DNA injection of the indicated vectors. (B–D) DNA vectors encoding FVIII-BDD or R593C-BDD (R593C) (27) were injected into tail vein. After 24 h, plasma and liver samples were harvested for TUNEL (B), FVIII antigen in plasma and liver (C), and real-time RT-PCR (D). A, C, and D depict three independent mice. (E) Protein misfolding and oxidative stress create a vicious cycle leading to ER stress and cell death. ROS are generated by exposure to multiple stresses and also as a byproduct of mitochondrial respiration. Protein misfolding may cause ROS through changes in oxidative phosphorylation as a consequence of energy depletion or Ca2+ release from the ER. In addition, GSH can be consumed to reduce improperly paired disulfide bonds within misfolded proteins. ROS production can interfere with protein folding by inactivating PDI/ERO1 thiol-disulfide exchange reactions and/or by causing aberrant disulfide bond formation. ER stress activates CHOP expression that may lead to ROS production through induction of Ero1 or Gadd34. In this model, ROS can cause protein misfolding, UPR activation, and CHOP induction.
Similar articles
- Factor VIII exhibits chaperone-dependent and glucose-regulated reversible amyloid formation in the endoplasmic reticulum.
Poothong J, Pottekat A, Siirin M, Campos AR, Paton AW, Paton JC, Lagunas-Acosta J, Chen Z, Swift M, Volkmann N, Hanein D, Yong J, Kaufman RJ. Poothong J, et al. Blood. 2020 May 21;135(21):1899-1911. doi: 10.1182/blood.2019002867. Blood. 2020. PMID: 32128578 Free PMC article. - Chemical chaperones improve protein secretion and rescue mutant factor VIII in mice with hemophilia A.
Roth SD, Schüttrumpf J, Milanov P, Abriss D, Ungerer C, Quade-Lyssy P, Simpson JC, Pepperkok R, Seifried E, Tonn T. Roth SD, et al. PLoS One. 2012;7(9):e44505. doi: 10.1371/journal.pone.0044505. Epub 2012 Sep 4. PLoS One. 2012. PMID: 22973456 Free PMC article. - Oxidative stress mediated Ca(2+) release manifests endoplasmic reticulum stress leading to unfolded protein response in UV-B irradiated human skin cells.
Farrukh MR, Nissar UA, Afnan Q, Rafiq RA, Sharma L, Amin S, Kaiser P, Sharma PR, Tasduq SA. Farrukh MR, et al. J Dermatol Sci. 2014 Jul;75(1):24-35. doi: 10.1016/j.jdermsci.2014.03.005. Epub 2014 Apr 13. J Dermatol Sci. 2014. PMID: 24794973 - Natural antioxidants' effects on endoplasmic reticulum stress-related diseases.
Reyes-Fermín LM, Aparicio-Trejo OE, Avila-Rojas SH, Gómez-Sierra T, Martínez-Klimova E, Pedraza-Chaverri J. Reyes-Fermín LM, et al. Food Chem Toxicol. 2020 Apr;138:111229. doi: 10.1016/j.fct.2020.111229. Epub 2020 Feb 24. Food Chem Toxicol. 2020. PMID: 32105807 Review. - Endoplasmic Reticulum Stress and Oxidative Stress: A Vicious Nexus Implicated in Bowel Disease Pathophysiology.
Chong WC, Shastri MD, Eri R. Chong WC, et al. Int J Mol Sci. 2017 Apr 5;18(4):771. doi: 10.3390/ijms18040771. Int J Mol Sci. 2017. PMID: 28379196 Free PMC article. Review.
Cited by
- The unfolded protein response and chemical chaperones reduce protein misfolding and colitis in mice.
Cao SS, Zimmermann EM, Chuang BM, Song B, Nwokoye A, Wilkinson JE, Eaton KA, Kaufman RJ. Cao SS, et al. Gastroenterology. 2013 May;144(5):989-1000.e6. doi: 10.1053/j.gastro.2013.01.023. Epub 2013 Jan 18. Gastroenterology. 2013. PMID: 23336977 Free PMC article. - NRF2-mediated signaling is a master regulator of transcription factors in bovine granulosa cells under oxidative stress condition.
Taqi MO, Saeed-Zidane M, Gebremedhn S, Salilew-Wondim D, Tholen E, Neuhoff C, Hoelker M, Schellander K, Tesfaye D. Taqi MO, et al. Cell Tissue Res. 2021 Sep;385(3):769-783. doi: 10.1007/s00441-021-03445-4. Epub 2021 May 19. Cell Tissue Res. 2021. PMID: 34008050 Free PMC article. - An involvement of oxidative stress in endoplasmic reticulum stress and its associated diseases.
Bhandary B, Marahatta A, Kim HR, Chae HJ. Bhandary B, et al. Int J Mol Sci. 2012 Dec 24;14(1):434-56. doi: 10.3390/ijms14010434. Int J Mol Sci. 2012. PMID: 23263672 Free PMC article. Review. - Life and death of proteins: a case study of glucose-starved Staphylococcus aureus.
Michalik S, Bernhardt J, Otto A, Moche M, Becher D, Meyer H, Lalk M, Schurmann C, Schlüter R, Kock H, Gerth U, Hecker M. Michalik S, et al. Mol Cell Proteomics. 2012 Sep;11(9):558-70. doi: 10.1074/mcp.M112.017004. Epub 2012 May 3. Mol Cell Proteomics. 2012. PMID: 22556279 Free PMC article. - Gene therapy - are we ready now?
Kaczmarek R. Kaczmarek R. Haemophilia. 2022 May;28 Suppl 4(Suppl 4):35-43. doi: 10.1111/hae.14530. Haemophilia. 2022. PMID: 35521736 Free PMC article.
References
- Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal. 2007;9:2277–2293. - PubMed
- Harding HP, et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11:619–633. - PubMed
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. - PubMed
- Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL057346/HL/NHLBI NIH HHS/United States
- DK042394/DK/NIDDK NIH HHS/United States
- HL052173/HL/NHLBI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R37 DK042394/DK/NIDDK NIH HHS/United States
- HL057346/HL/NHLBI NIH HHS/United States
- R01 DK042394/DK/NIDDK NIH HHS/United States
- R01 HL052173/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical