The role of nicotine in smoking: a dual-reinforcement model - PubMed (original) (raw)
The role of nicotine in smoking: a dual-reinforcement model
Anthony R Caggiula et al. Nebr Symp Motiv. 2009.
Abstract
Models of intravenous nicotine self-administration in laboratory animals are being used to investigate the behavioral and neurobiological consequences of nicotine reinforcement, and to aid in the development of novel pharmacotherapies for smoking cessation. Central to these models is the principle of primary reinforcement, which posits that response-contingent presentation of a primary reinforcer, nicotine, engenders robust operant behavior, whereas response-independent drug delivery does not. This dictum of nicotine as a primary reinforcer has been widely used to explain why people smoke tobacco-smoking results in the rapid delivery of nicotine to the brain, setting up a cascade of neurobiological processes that strengthen subsequent smoking behavior. However, there is mounting evidence that the primary reinforcement model of nicotine self-administration fails to fully explain existing data from both the animal self-administration and human smoking literatures. We have recently proposed a "dual reinforcement" model to more fully capture the relationship between nicotine and self-administration, including smoking. Briefly, the "dual reinforcement" model posits that nicotine acts as both a primary reinforcer and a reinforcement enhancer. The latter action of nicotine had originally been uncovered by showing that a reinforcing VS, which accompanies nicotine delivery, synergizes with nicotine in the acquisition and maintenance of self-administration, and that this synergism can be reproduced by combining operant responding for the reinforcing stimulus with non-contingent (response-independent) nicotine. Thus, self-administration (and smoking) is sustained by three actions: (1) nicotine, acting as a primary reinforcer, can sustain behavior that leads to its delivery; (2) nicotine, acting as a primary reinforcer, can establish neutral environmental stimuli as conditioned reinforcers through Pavlovian associations; and (3) nicotine, acting as a reinforcement enhancer, can magnify the incentive value of accompanying stimuli, be they conditioned or unconditioned reinforcers.
Figures
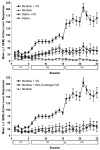
Figure 6.1
Mean (+SE) responses on the active lever for NIC+VS peaks at more than twice the sum of NIC alone plus VS alone. For clarity the lower panel reproduces the NIC and NIC + VS data of the upper panel and shows non-contingent VS exposure (controlled by rats in the NIC+VS group through yoking) does not synergize with contingent NIC. N=8-12/group. Data derived from Donny et al (2003).
Figure 6.2
Effects of response-contingent, yoked, or continuous nicotine or responding for VS. Acquisition data (days1-20) were followed by 6 days in which saline was substituted for nicotine (days 21-26) and then three days in which nicotine was replaced (days 27-29). Results are mean + SEM of data obtained from 7-9 animals per group (From Donny et al. 2003. Copyright © 2003 by Springer-Verlag. Reprinted with permission)
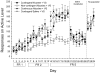
Figure 6.3
(a) Responses on the active lever, inactive lever and number of stimulus presentations (mean + SEM) during a 30-minute test for conditioned reinforcement by rats trained previously with a stimulus that was paired with sucrose (n=41), or the same stimulus that was explicitly unpaired with sucrose (n=25). (b) Interaction between stimulus-training condition (sucrose-paired vs. sucrose-unpaired) and drug contingency (contingent NIC vs. non-contingent NIC). Data from non-contingent SAL + stimulus conditions are also shown for comparison. Panels represent data from either the entire 180 minute test session (no break point), or after a 30 minute or 60 minute break point was imposed. Data are mean (* SEM) stimulus presentations earned on the last 2 days of the progressive ratio schedule. All interactions are significant at P<0.05 (From Chaudhri et al. 2006a. Copyright © 2006 by Springer-Verlag. Reprinted with permission)
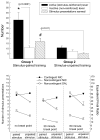
Figure 6.4
Left panel shows the mean (±1 SEM) number of active- and inactive-lever responses made during the stimulus comparison phase. Active-lever responses were significantly higher in the House-Light off than the Lever-Light on condition. Right panel illustrates the mean (±1 SEM) active-lever responses made during nicotine/saline testing sessions. Active-lever responding in House-Light NIC condition was significantly greater than the House-Light Sal condition. The number of reinforcements earned in this study was previously reported in Palmatier et al. (2007).
Figure 6.5
Mean (+1 SEM) active lever responding for rats in the VS-Only, NIC-Only, NIC+VS and 2-Lever groups. For the 2-Lever group, responding on the infusion lever (■) is depicted separately from responding on the VS lever (□). Time-out responding is excluded. For the 2-Lever group, responding for the VS lever exceeded responding in the VS-Only group for FR2 sessions and did not differ from VS+NIC rats. Saline substitution abolished, and NIC replacement reinstated the difference between 2-Lever VS and VS-Only responding. See Palmatier et al, 2006 for details of procedures and statistical analyses. (From Palmatier et al. 2006. Copyright © 2005 by Springer-Verlag. Reprinted with permission)
Similar articles
- Operant responding for conditioned and unconditioned reinforcers in rats is differentially enhanced by the primary reinforcing and reinforcement-enhancing effects of nicotine.
Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, Palmatier MI, Liu X, Sved AF. Chaudhri N, et al. Psychopharmacology (Berl). 2006 Nov;189(1):27-36. doi: 10.1007/s00213-006-0522-0. Epub 2006 Sep 22. Psychopharmacology (Berl). 2006. PMID: 17019569 - Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement.
Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF. Chaudhri N, et al. Psychopharmacology (Berl). 2006 Mar;184(3-4):353-66. doi: 10.1007/s00213-005-0178-1. Epub 2005 Oct 21. Psychopharmacology (Berl). 2006. PMID: 16240165 Review. - The reinforcement enhancing effects of nicotine depend on the incentive value of non-drug reinforcers and increase with repeated drug injections.
Palmatier MI, Matteson GL, Black JJ, Liu X, Caggiula AR, Craven L, Donny EC, Sved AF. Palmatier MI, et al. Drug Alcohol Depend. 2007 Jun 15;89(1):52-9. doi: 10.1016/j.drugalcdep.2006.11.020. Epub 2007 Jan 19. Drug Alcohol Depend. 2007. PMID: 17240084 Free PMC article. - Dissociating the primary reinforcing and reinforcement-enhancing effects of nicotine using a rat self-administration paradigm with concurrently available drug and environmental reinforcers.
Palmatier MI, Evans-Martin FF, Hoffman A, Caggiula AR, Chaudhri N, Donny EC, Liu X, Booth S, Gharib M, Craven L, Sved AF. Palmatier MI, et al. Psychopharmacology (Berl). 2006 Mar;184(3-4):391-400. doi: 10.1007/s00213-005-0183-4. Epub 2005 Oct 25. Psychopharmacology (Berl). 2006. PMID: 16249908 - Sex differences in nicotine reinforcement and reward: influences on the persistence of tobacco smoking.
Perkins KA. Perkins KA. Nebr Symp Motiv. 2009;55:143-69. doi: 10.1007/978-0-387-78748-0_9. Nebr Symp Motiv. 2009. PMID: 19013943 Review. No abstract available.
Cited by
- A quantitative analysis of the reward-enhancing effects of nicotine using reinforcer demand.
Barrett ST, Bevins RA. Barrett ST, et al. Behav Pharmacol. 2012 Dec;23(8):781-9. doi: 10.1097/FBP.0b013e32835a38d9. Behav Pharmacol. 2012. PMID: 23080311 Free PMC article. - The effect of nicotine on sign-tracking and goal-tracking in a Pavlovian conditioned approach paradigm in rats.
Palmatier MI, Marks KR, Jones SA, Freeman KS, Wissman KM, Sheppard AB. Palmatier MI, et al. Psychopharmacology (Berl). 2013 Mar;226(2):247-59. doi: 10.1007/s00213-012-2892-9. Epub 2012 Oct 23. Psychopharmacology (Berl). 2013. PMID: 23090624 - Individual variation in resisting temptation: implications for addiction.
Saunders BT, Robinson TE. Saunders BT, et al. Neurosci Biobehav Rev. 2013 Nov;37(9 Pt A):1955-75. doi: 10.1016/j.neubiorev.2013.02.008. Epub 2013 Feb 21. Neurosci Biobehav Rev. 2013. PMID: 23438893 Free PMC article. Review. - The effects of response operandum and prior food training on intravenous nicotine self-administration in rats.
Clemens KJ, Caillé S, Cador M. Clemens KJ, et al. Psychopharmacology (Berl). 2010 Jul;211(1):43-54. doi: 10.1007/s00213-010-1866-z. Epub 2010 May 1. Psychopharmacology (Berl). 2010. PMID: 20437028 - The likelihood of khat chewing serving as a neglected and reverse 'gateway' to tobacco use among UK adult male khat chewers: a cross sectional study.
Kassim S, Rogers N, Leach K. Kassim S, et al. BMC Public Health. 2014 May 13;14:448. doi: 10.1186/1471-2458-14-448. BMC Public Health. 2014. PMID: 24885131 Free PMC article.
References
- Arroyo M, Markou A, Robbins TW, Everitt BJ. Acquisition, maintenance and reinstatement of intravenous cocaine self-administration under a second-order schedule of reinforcement in rats: effects of conditioned cues and continuous access to cocaine. Psychopharmacology (Berl) 1998;140(3):331–344. - PubMed
- Balfour DJ, Wright AE, Benwell ME, Birrell CE. The putative role of extra-synaptic mesolimbic dopamine in the neurobiology of nicotine dependence. Behav Brain Res. 2000;113(12):73–83. - PubMed
- Bevins RA, Palmatier MI. Extending the role of associative learning processes in nicotine addiction. Behavioral and Cognitive Neuroscience Review. 2004;3:143–158. - PubMed
- Bozarth MA, Murray A, Wise RA. Influence of housing conditions on the acquisition of intravenous heroin and cocaine self-administration in rats. Pharmacol Biochem Behav. 1989;33(4):903–907. - PubMed
- Brunzell DH, Chang JR, Schneider B, Olausson P, Taylor JR, Picciotto MR. beta2-Subunit-containing nicotinic acetylcholine receptors are involved in nicotine-induced increases in conditioned reinforcement but not progressive ratio responding for food in C57BL/6 mice. Psychopharmacology (Berl) 2006;184(34):328–338. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DA010464/DA/NIDA NIH HHS/United States
- R01 DA017288/DA/NIDA NIH HHS/United States
- DA-12655/DA/NIDA NIH HHS/United States
- DA-10464/DA/NIDA NIH HHS/United States
- DA-17288/DA/NIDA NIH HHS/United States
- DA-19278/DA/NIDA NIH HHS/United States
- R01 DA012655/DA/NIDA NIH HHS/United States
- F32 DA019278/DA/NIDA NIH HHS/United States
- R01 DA010464-12A1/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources