Both telomeric and non-telomeric DNA damage are determinants of mammalian cellular senescence - PubMed (original) (raw)
Both telomeric and non-telomeric DNA damage are determinants of mammalian cellular senescence
Asako J Nakamura et al. Epigenetics Chromatin. 2008.
Abstract
Background: Cellular senescence is a state reached by normal mammalian cells after a finite number of cell divisions and is characterized by morphological and physiological changes including terminal cell-cycle arrest. The limits on cell division imposed by senescence may play an important role in both organismal aging and in preventing tumorigenesis. Cellular senescence and organismal aging are both accompanied by increased DNA damage, seen as the formation of gamma-H2AX foci (gamma-foci), which may be found on uncapped telomeres or at non-telomeric sites of DNA damage. However, the relative importance of telomere- and non-telomere-associated DNA damage to inducing senescence has never been demonstrated. Here we present a new approach to determine accurately the chromosomal location of gamma-foci and quantify the number of telomeric versus non-telomeric gamma-foci associated with senescence in both human and mouse cells. This approach enables researchers to obtain accurate values and to avoid various possible misestimates inherent in earlier methods.
Results: Using combined immunofluorescence and telomere fluorescence in situ hybridization on metaphase chromosomes, we show that human cellular senescence is not solely determined by telomeric DNA damage. In addition, mouse cellular senescence is not solely determined by non-telomeric DNA damage. By comparing cells from different generations of telomerase-null mice with human cells, we show that cells from late generation telomerase-null mice, which have substantially short telomeres, contain mostly telomeric gamma-foci. Most notably, we report that, as human and mouse cells approach senescence, all cells exhibit similar numbers of total gamma-foci per cell, irrespective of chromosomal locations.
Conclusion: Our results suggest that the chromosome location of senescence-related gamma-foci is determined by the telomere length rather than species differences per se. In addition, our data indicate that both telomeric and non-telomeric DNA damage responses play equivalent roles in signaling the initiation of cellular senescence and organismal aging. These data have important implications in the study of mechanisms to induce or delay cellular senescence in different species.
Figures
Figure 1
γ-H2AX immunostaining on metaphase chromosomes. (A) Typical metaphase spreads of human (left) and mouse (right) fibroblasts stained for γ-H2AX (green) and telomeric DNA (red). (B) Scoring of foci as along the chromatid arms, proximal or distal to the telomeres, or on the chromatid ends, fluorescence in situ hybridization negative or positive.
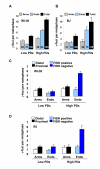
Figure 2
Distribution of senescence-related γ-foci in human fibroblasts. (A-B) Distribution of γ-foci on the metaphases of low and high population doublings (PDs) (pre-senescent), human embryo lung fibroblast (WI-38) (A) and foreskin fibroblast (BJ) (B). Proportion (%) of each type of damage is shown in each graph bar. Scoring is as in Figure 1B. (C-D) Scoring of the γ-foci as along the chromatid arms proximal to the telomere, along the chromatid arms distal to the telomeres, on the chromatid ends with fluorescence in situ hybridization (FISH) signal or on the chromatid ends without FISH signal. Low and high PDs of WI-38 cells (C) and BJ cells (D). On average more than 10 metaphases were screened per point in independent experiments. Error bars signify standard errors.
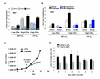
Figure 3
Distribution of senescence-related γ-foci in mouse embryo fibroblasts. (A) Distribution of γ-foci on metaphases of primary mouse embryonic fibroblasts (MEF), low population doublings (PDs), high PDs in 20% O2 and 3% O2 (see Additional file 1 for experimental details). Proportion (%) of each type of damage is shown in each graph bar. Scoring is as in Figure 1B. (B) Scoring of γ-foci as along the chromatid arms proximal to telomere, along the chromatid arms distal to the telomeres, on the chromatid ends with fluorescence in situ hybridization (FISH) signal or on the chromatid ends without FISH signal. On average more than 10 metaphases were screened per point in independent experiments. (C) MEFs cultured in 20% O2 (triangle) were shifted at day 5 to 3% O2 (square), or maintained in 20% O2. The average of two cultures is shown. Note that the cultures transferred from 20% to 3% O2 in panel A were already pre-senescent, and also became senescent in 3% O2. The cultures transferred from 20% to 3% O2 in this panel were low PDs and accelerated growth in 3% O2. (D) Number of γ-foci in MEFs after oxygen transfer is shown. See Additional file 3 for experimental details. Error bars signify standard errors.
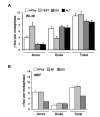
Figure 4
Origins of senescence-associated γ-foci. (A) Distribution of γ-foci in high population doubling (PD) WI-38 cells, after the expression of telomerase (TERT), after culturing with the 50 μM antioxidant tempol (OX), or in VA-13 with alternative lengthening of telomeres (ALT). (B) Distribution of γ-foci in high PD mouse embryonic fibroblast (MEF) cells, in spontaneously immortalized MEF cells (IM) or after culturing with the 50 μM tempol (OX). Scoring is as in Figure 1B. On average more than 10 metaphases were screened per point in independent experiments. Error bars signify standard errors.
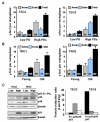
Figure 5
Effect of telomerase deficiency on the distribution of senescence-related γ-foci and activation of damage signaling. (A) Distribution of γ-foci in low population doubling (PD) and pre-senescent mouse embryonic fibroblasts (MEFs) from second (TEG2) and fifth (TEG5) generation telomere reverse transcriptase (TERT)-null mice. (B) Distribution of γ-foci in splenic lymphocytes taken from young and old first (TRG1) generation mTR-null mice, and from young and old fifth (TRG5) generation mTR-null mice. Scoring is as in Figure 1B. On average more than 10 metaphases were screened per point in independent experiments. Error bars signify standard errors. (C) Levels of DNA damage checkpoint proteins in early and high PDs MEFs from second (TEG2) and fifth (TEG5) generation TERT-null mice. Relative induction in senescent compared with low PD cells of the proteins shown in the left panel. Dashed line denotes no induction.
Figure 6
Senescence in mammalian cells is associated with a set number of γ-foci regardless of origin. (A) Numbers of γ-foci in the high population doublings or old cultures of the primary cell strains are arranged to show that the average total numbers of γ-foci per senescent or old cell are in the range 8–11, while the numbers of γ-foci found on the chromosome ends or on the arms are more variable. (B) The fractions of metaphase cells containing each range of γ-focal numbers are presented. Ranges of γ-focal numbers are taken to smooth the data.
Similar articles
- Telomere-dependent and telomere-independent origins of endogenous DNA damage in tumor cells.
Nakamura AJ, Redon CE, Bonner WM, Sedelnikova OA. Nakamura AJ, et al. Aging (Albany NY). 2009 Feb 4;1(2):212-8. doi: 10.18632/aging.100019. Aging (Albany NY). 2009. PMID: 20157510 Free PMC article. - Premature senescence of mesothelial cells is associated with non-telomeric DNA damage.
Ksiazek K, Passos JF, Olijslagers S, Saretzki G, Martin-Ruiz C, von Zglinicki T. Ksiazek K, et al. Biochem Biophys Res Commun. 2007 Oct 26;362(3):707-11. doi: 10.1016/j.bbrc.2007.08.047. Epub 2007 Aug 20. Biochem Biophys Res Commun. 2007. PMID: 17720141 - Delayed kinetics of DNA double-strand break processing in normal and pathological aging.
Sedelnikova OA, Horikawa I, Redon C, Nakamura A, Zimonjic DB, Popescu NC, Bonner WM. Sedelnikova OA, et al. Aging Cell. 2008 Jan;7(1):89-100. doi: 10.1111/j.1474-9726.2007.00354.x. Epub 2007 Dec 19. Aging Cell. 2008. PMID: 18005250 - The role of telomeres and telomerase in the pathology of human cancer and aging.
Shin JS, Hong A, Solomon MJ, Lee CS. Shin JS, et al. Pathology. 2006 Apr;38(2):103-13. doi: 10.1080/00313020600580468. Pathology. 2006. PMID: 16581649 Review. - DNA repair factors and telomere-chromosome integrity in mammalian cells.
Hande MP. Hande MP. Cytogenet Genome Res. 2004;104(1-4):116-22. doi: 10.1159/000077475. Cytogenet Genome Res. 2004. PMID: 15162024 Review.
Cited by
- Ionizing radiation-induced foci persistence screen to discover enhancers of accelerated senescence.
Labay E, Efimova EV, Quarshie BK, Golden DW, Weichselbaum RR, Kron SJ. Labay E, et al. Int J High Throughput Screen. 2011 Mar;2:1-13. doi: 10.2147/IJHTS.S17076. Int J High Throughput Screen. 2011. PMID: 26097382 Free PMC article. - EML4-ALK induces cellular senescence in mortal normal human cells and promotes anchorage-independent growth in hTERT-transduced normal human cells.
Miyanaga A, Matsumoto M, Beck JA, Horikawa I, Oike T, Okayama H, Tanaka H, Burkett SS, Robles AI, Khan M, Lissa D, Seike M, Gemma A, Mano H, Harris CC. Miyanaga A, et al. BMC Cancer. 2021 Mar 24;21(1):310. doi: 10.1186/s12885-021-07905-6. BMC Cancer. 2021. PMID: 33761896 Free PMC article. - Focus on histone variant H2AX: to be or not to be.
Yuan J, Adamski R, Chen J. Yuan J, et al. FEBS Lett. 2010 Sep 10;584(17):3717-24. doi: 10.1016/j.febslet.2010.05.021. Epub 2010 May 21. FEBS Lett. 2010. PMID: 20493860 Free PMC article. Review. - Modulation of the ATM/autophagy pathway by a G-quadruplex ligand tips the balance between senescence and apoptosis in cancer cells.
Beauvarlet J, Bensadoun P, Darbo E, Labrunie G, Rousseau B, Richard E, Draskovic I, Londono-Vallejo A, Dupuy JW, Nath Das R, Guédin A, Robert G, Orange F, Croce S, Valesco V, Soubeyran P, Ryan KM, Mergny JL, Djavaheri-Mergny M. Beauvarlet J, et al. Nucleic Acids Res. 2019 Apr 8;47(6):2739-2756. doi: 10.1093/nar/gkz095. Nucleic Acids Res. 2019. PMID: 30759257 Free PMC article. - Premature aging.
Vulliamy TJ. Vulliamy TJ. Cell Mol Life Sci. 2009 Sep;66(18):3091-4. doi: 10.1007/s00018-009-0091-6. Epub 2009 Jul 18. Cell Mol Life Sci. 2009. PMID: 19618112 Free PMC article. Review. No abstract available.
References
LinkOut - more resources
Full Text Sources