Mechanisms for human genomic rearrangements - PubMed (original) (raw)
Mechanisms for human genomic rearrangements
Wenli Gu et al. Pathogenetics. 2008.
Abstract
Genomic rearrangements describe gross DNA changes of the size ranging from a couple of hundred base pairs, the size of an average exon, to megabases (Mb). When greater than 3 to 5 Mb, such changes are usually visible microscopically by chromosome studies. Human diseases that result from genomic rearrangements have been called genomic disorders. Three major mechanisms have been proposed for genomic rearrangements in the human genome. Non-allelic homologous recombination (NAHR) is mostly mediated by low-copy repeats (LCRs) with recombination hotspots, gene conversion and apparent minimal efficient processing segments. NAHR accounts for most of the recurrent rearrangements: those that share a common size, show clustering of breakpoints, and recur in multiple individuals. Non-recurrent rearrangements are of different sizes in each patient, but may share a smallest region of overlap whose change in copy number may result in shared clinical features among different patients. LCRs do not mediate, but may stimulate non-recurrent events. Some rare NAHRs can also be mediated by highly homologous repetitive sequences (for example, Alu, LINE); these NAHRs account for some of the non-recurrent rearrangements. Other non-recurrent rearrangements can be explained by non-homologous end-joining (NHEJ) and the Fork Stalling and Template Switching (FoSTeS) models. These mechanisms occur both in germ cells, where the rearrangements can be associated with genomic disorders, and in somatic cells in which such genomic rearrangements can cause disorders such as cancer. NAHR, NHEJ and FoSTeS probably account for the majority of genomic rearrangements in our genome and the frequency distribution of the three at a given locus may partially reflect the genomic architecture in proximity to that locus. We provide a review of the current understanding of these three models.
Figures
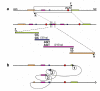
Figure 1
Experimental observations of recurrent and non-recurrent genomic rearrangements associated with genomic disorders. The long thin line signifies the genomic region undergoing genomic rearrangements. The black rectangle depicts a gene which is located in the rearranged region and can be affected by the rearrangements. The thick blue (in a) and red (in b and c) bars represent the rearrangements (duplications, deletions or inversions) with their breakpoints a. recurrent rearrangements with a common size and clustered breakpoints. Most of the recurrent rearrangements result from non-allelic homologous recombination (NAHR). The two hatched rectangles flanking the gene depict the low-copy repeats (LCRs) functioning as substrates for NAHR. The rearrangement breakpoints are clustered inside the LCRs. b. Non-recurrent rearrangements. The breakpoints of the non-recurrent rearrangements are scattered in the genomic region. Note, that all of the rearrangements share a common genomic region of overlap, the smallest region of overlap, that encompasses a gene necessary for the conveyed phenotypic trait, which enables these rearrangements to be ascertained. c. Non-recurrent rearrangements with grouping of one breakpoint. Some of the non-recurrent rearrangements have one of their breakpoints localized in one small genomic region. This grouping of breakpoints is distinct from breakpoint clustering, but like clustering, it may reflect underlying genomic architecture (for example, palindrome or cruciform) important to the rearrangement mechanism, depicted as the dotted rectangle in Figure 1c.
Figure 2
Genomic rearrangements (Adapted from [2] and [5]). a1 and a2 Genomic rearrangements resulting from recombination between low-copy repeats (LCRs). LCRs are depicted as black arrows with the orientation indicated by the direction of the arrowhead. Capital letters above the thin horizontal lines refer to the flanking unique sequences (for example, A). Homologues on the other strand (can be another chromatid or the homologous chromosome) are also shown (for example, a). Thin diagonal lines refer to a recombination event with the results shown by numbers 1, 2 and 3. a1 Recombination between direct repeats results in deletion and/or duplication. a2 Recombination between inverted repeats results in an inversion. b. Schematic representation of reciprocal duplications and deletions mediated by interchromosomal (left), interchromatid (middle) and intrachromatid (right) non-allelic homologous recombination (NAHR) using LCR pairs in direct orientation. Chromosomes are shown in black, with the centromere depicted by hashed lines. Yellow arrows depict LCRs. Letters adjacent to the chromatids refer to the flanking unique sequence (for example, A, a). Interchromosomal and interchromatid NAHR between LCRs in direct orientation result in reciprocal duplication and deletion, whereas intrachromatid NAHR only creates deletion. Signatures of homologous recombination include the sequence identity of the substrates (LCRs) used for NAHR, recombination hotspots within the LCRs, and evidence for gene conversion at the crossovers within the LCRs.
Figure 3
Genomic rearrangement mechanisms. a. (Adapted from [66]) Non-homologous end-joining (NHEJ) in vertebrates. A double-stranded DNA break (DSB) occurs and is repaired via NHEJ mechanism. The two thick lines depict two DNA strands with DSB, the thin segments in the middle represent the modifications which the ends have gone through before the final ligation. The enzyme machineries catalyzing each step are briefly summarized. They are described in details in references [65] and [70]. Note at step 3 that in order to repair ends, some addition or deletion of bases may be required, leaving behind a 'signature' of NHEJ. b. (Adapted from [82]) After the original stalling of the replication fork (dark blue and red, solid lines), the lagging strand (red, dotted line) disengages and anneals to a second fork (purple and green, solid lines) via microhomology (1), followed by (2) extension of the now 'primed' second fork and DNA synthesis (green, dotted line). After the fork disengages (3), the tethered original fork (dark blue and red, solid lines) with its lagging strand (red and green, dotted lines) could invade a third fork (gray and black, solid lines). Dotted lines represent newly synthesized DNA. Serial replication fork disengaging and lagging strand invasion could occur several times (e.g. FoSTeS x 2, FoSTeS x 3, ... etc.) before (4) resumption of replication on the original template.
Figure 4
One Pelizaeus-Merzbacher disease (PMD)-associated complex PLP1 rearrangement results from multiple FoSTeS events, FoSTeS × 3 (Adapted from [82]). a. Duplication junctions (vertical dotted lines) for one PMD patient are displayed relative to reference sequence, with the duplicated region boxed. Two or three base pairs of microhomology were found at the breakpoint junctions (i.e. "joint points") after amplification with outward-facing primers (F and R). b. Illustration of the order, origins, and relative orientations of junctional (pink and blue) and boundary reference sequences (orange and green) for the PMD patient. Arrowheads show direction of DNA relative to the positive strand; filled arrowheads with circled numbers below represent a FoSTeS event; open arrowhead marks resumption of replication on the original template. Proximal (centromeric) and distal (telomeric) are in relation to PLP1 (red circle).
Figure 5
Comparison of non-allelic homologous recombination, non-homologous end-joining and Fork Stalling and Template Switching mechanisms resulting in genomic duplication/deletion. The two thin lines in all three schemes represent the double strands of DNA. Left column: An intrachromatid non-allelic homologous recombination (NAHR) event. Rectangles in different shades of blue depict two directly orientated low-copy reapeats (LCRs) sharing high homology (97% to 98%), which align at non-allelic rather than allelic positions and the subsequent recombination causes deletion or duplication (reciprocal events but not with equivalent frequencies) of part of the two LCRs as well as the segment flanked by them. Middle column: a non-homologous end-joining (NHEJ) event. Double-strand breaks (DSBs) are created between the two sequences represented as a blue and a red rectangle with no homology between each other. The NHEJ system modifies and rejoins the two ends, resulting in the deletion of the segment between the two DSBs. Right column: a Fork Stalling and Template Switching (FoSTeS) × 2 event causing a complex deletion involving two fragments. No extensive homology is required between the substrate sequences depicted by a blue, a red and a green rectangle. However, the small open triangle heading downwards depicts a site bearing microhomology (2 to 5 base pairs) between the blue and the red sequences, and the small filled triangle heading downwards depicts another site bearing microhomology between the red and the green sequences. Different from NAHR and NHEJ, the FoSTeS event occurs during DNA replication. The replication forks from the two surrounding sequences are shown in the same color as the rectangles. The leading nascent strand at the left side (blue or red) fork invades the right side (red or green) fork via the demonstrated microhomology, and primes its own further synthesis using the right side fork as template. This event happens twice, causing deletion of the two fragments flanked by each pair of microhomology sites. Note the juxtaposition of genomic sequences from multiple distinct regions yielding complex rearrangements.
Figure 6
Genomic architecture is crucial for the genomic rearrangements. The low-copy repeats serve as substrates and thus are an indispensable requirement of non-allelic homologous recombination. Current data suggest that local genomic architecture, including palindromes or cruciforms, might be a stimulus for the Fork Stalling and Template Switching (FoSTeS) rearrangement as well, although these architectural elements are not necessarily directly involved in the FoSTeS rearrangement per se. This could account for the observation of breakpoint grouping with non-recurrent rearrangements at some loci.
Similar articles
- Decoding NF1 Intragenic Copy-Number Variations.
Hsiao MC, Piotrowski A, Callens T, Fu C, Wimmer K, Claes KB, Messiaen L. Hsiao MC, et al. Am J Hum Genet. 2015 Aug 6;97(2):238-49. doi: 10.1016/j.ajhg.2015.06.002. Epub 2015 Jul 16. Am J Hum Genet. 2015. PMID: 26189818 Free PMC article. - Mechanisms of structural chromosomal rearrangement formation.
Burssed B, Zamariolli M, Bellucco FT, Melaragno MI. Burssed B, et al. Mol Cytogenet. 2022 Jun 14;15(1):23. doi: 10.1186/s13039-022-00600-6. Mol Cytogenet. 2022. PMID: 35701783 Free PMC article. Review. - Genomic disorders: molecular mechanisms for rearrangements and conveyed phenotypes.
Lupski JR, Stankiewicz P. Lupski JR, et al. PLoS Genet. 2005 Dec;1(6):e49. doi: 10.1371/journal.pgen.0010049. PLoS Genet. 2005. PMID: 16444292 Free PMC article. Review. - Human endogenous retroviral elements promote genome instability via non-allelic homologous recombination.
Campbell IM, Gambin T, Dittwald P, Beck CR, Shuvarikov A, Hixson P, Patel A, Gambin A, Shaw CA, Rosenfeld JA, Stankiewicz P. Campbell IM, et al. BMC Biol. 2014 Sep 23;12:74. doi: 10.1186/s12915-014-0074-4. BMC Biol. 2014. PMID: 25246103 Free PMC article.
Cited by
- Template switching during DNA replication is a prevalent source of adaptive gene amplification.
Chuong JN, Nun NB, Suresh I, Matthews JC, De T, Avecilla G, Abdul-Rahman F, Brandt N, Ram Y, Gresham D. Chuong JN, et al. bioRxiv [Preprint]. 2024 Oct 15:2024.05.03.589936. doi: 10.1101/2024.05.03.589936. bioRxiv. 2024. PMID: 39464144 Free PMC article. Preprint. - DNA methylation dysregulation patterns in the 1p36 region instability.
Swierkowska-Janc J, Kabza M, Rydzanicz M, Giefing M, Ploski R, Shaffer LG, Gajecka M. Swierkowska-Janc J, et al. J Appl Genet. 2024 Oct 26. doi: 10.1007/s13353-024-00913-9. Online ahead of print. J Appl Genet. 2024. PMID: 39460848 - UHRF1-mediated ubiquitination of nonhomologous end joining factor XLF promotes DNA repair in human tumor cells.
Deng Z, Long C, Han S, Xu Z, Hou T, Li W, Wang X, Liu X. Deng Z, et al. J Biol Chem. 2024 Sep 27;300(11):107823. doi: 10.1016/j.jbc.2024.107823. Online ahead of print. J Biol Chem. 2024. PMID: 39341501 Free PMC article. - Isolation and Characterization of High-Temperature-Tolerant Mutants of Bradyrhizobium diazoefficiens USDA110 by Carbon-Ion Beam Irradiation.
Satoh K, Takeda K, Nagafune I, Chik WDW, Ohkama-Otsu N, Okazaki S, Yokoyama T, Hase Y. Satoh K, et al. Microorganisms. 2024 Sep 2;12(9):1819. doi: 10.3390/microorganisms12091819. Microorganisms. 2024. PMID: 39338493 Free PMC article. - Gonadal Mosaicism as a Rare Inheritance Pattern in Recessive Genodermatoses: Report of Two Cases with Pseudoxanthoma Elasticum and Literature Review.
Dangreau L, Hosen MJ, De Zaeytijd J, Leroy BP, Coucke PJ, Vanakker OM. Dangreau L, et al. Curr Issues Mol Biol. 2024 Sep 11;46(9):9998-10007. doi: 10.3390/cimb46090597. Curr Issues Mol Biol. 2024. PMID: 39329949 Free PMC article.
References
- Lupski JR, Stankiewicz P. Genomic Disorders. Totowa, New Jersey: Humana Press; 2006.
LinkOut - more resources
Full Text Sources
Miscellaneous