Synaptic ultrastructural alterations anticipate the development of neuroaxonal dystrophy in sympathetic ganglia of aged and diabetic mice - PubMed (original) (raw)
Synaptic ultrastructural alterations anticipate the development of neuroaxonal dystrophy in sympathetic ganglia of aged and diabetic mice
Robert E Schmidt et al. J Neuropathol Exp Neurol. 2008 Dec.
Abstract
Neuroaxonal dystrophy, a distinctive axonopathy characterized by marked enlargement of distal axons, is the hallmark pathologic alteration in aged and diabetic human prevertebral sympathetic ganglia and in corresponding rodent models. Neuroaxonal dystrophy is thought to represent the abnormal outcome of cycles of synaptic degeneration and regeneration; a systematic study of identified axon terminals in aged and diabetic prevertebral ganglia, however, has not previously been performed. We examined the initial changes that develop in presynaptic and postsynaptic elements in sympathetic ganglia of aged and diabetic mice and found numerous synaptic changes involving both presynaptic and postsynaptic elements. Early alterations in presynaptic axon terminal size, vesicle content, and morphology culminate in the development of anastomosing membranous tubulovesicular aggregates, accumulation of autophagosomes, and amorphous debris that form a continuum with progressively larger classically dystrophic swellings. Dendritic changes consist of the development of swellings composed of delicate tubulovesicular elements and mitochondriopathy characterized by increased numbers of small mitochondria and, exclusively in aged ganglia, megamitochondria. These results support the hypothesis that neuroaxonal dystrophy results from progressive changes in presynaptic axon terminals that likely involve membrane dynamics and which are accompanied by distinctive changes in postsynaptic dendritic elements.
Figures
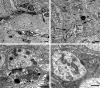
Figure 1
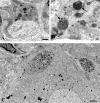
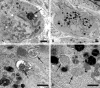
Normal young mouse SMG-CG ultrastructure. (A, B) A principal sympathetic neuron is surrounded by presynaptic axon terminals, dendrites (asterisk, B) and dendritic spines composing the neuropil. One area (arrow, A) is shown at higher magnification in B and consists of several presynaptic axon terminals (arrows, B) enclosed in satellite cell processes. Magnification bars: A, 2 μm; B, 500 nm. (C) A typical synapse shows a presynaptic active zone (arrow) with clusters of agranular synaptic vesicles, a postsynaptic density in a small dendrite (*) and rare dense core vesicles. Magnification bar: 500 nm. (D) A typical small dendrite with lucent cytoplasm (*) is immediately postsynaptic to a nerve terminal. Magnification bar: 500 nm.
Figure 2
Aged and young mouse SMG-CG presynaptic axon terminal cross sectional area size-frequency histogram.
Figure 3
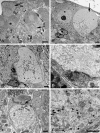
Presynaptic axon terminals of aged mouse SMG-CG. (A) Presynaptic nerve terminals in aged SMG-CG are typically larger than young controls, with numerous synaptic vesicles frequently without separation of a reserve from active zone, seen adjacent to a postsynaptic dendrite (*). Magnification bar: 500 nm. (B) Abnormal presynaptic terminals may be convoluted in shape (arrow) with several separate active zones. Magnification bar: 500 nm. (C, D) In addition to small, agranular synaptic vesicles and dense core vesicles, this axon terminal contains heterogeneously enlarged vesicles, seen at higher magnification in D (arrows, C, D), adjacent to a small dendrite (*, C). Magnification bars: C, 500 nm; D, 100 nm.
Figure 4
Accumulation of delicate tubulovesicular elements in presynaptic axon terminals in aged SMG-CG. (A–C) Presynaptic nerve terminals show anastomosing tubulovesicular elements (arrows) in close apposition to the active zone and admixed with agranular synaptic vesicles. Magnification bars: A, 500 nm; B, 100 nm; C, 500 nm. (D, E) Intimate association of agranular and heterogeneous agranular elements with tubulovesicular elements. Magnification bars: D, E, 500 nm. (F) Tubulovesicles may form connections with the axolemma (arrow). Magnification bar: 500 nm. (G) Progressive enlargement of presynaptic nerve terminals is dominated by delicate tubulovesicular elements. Magnification bar: 500 nm. (H) Typical dystrophic axon (arrow) is large with compact tubulovesicular elements. Magnification bar: 2 μm.
Figure 4
Accumulation of delicate tubulovesicular elements in presynaptic axon terminals in aged SMG-CG. (A–C) Presynaptic nerve terminals show anastomosing tubulovesicular elements (arrows) in close apposition to the active zone and admixed with agranular synaptic vesicles. Magnification bars: A, 500 nm; B, 100 nm; C, 500 nm. (D, E) Intimate association of agranular and heterogeneous agranular elements with tubulovesicular elements. Magnification bars: D, E, 500 nm. (F) Tubulovesicles may form connections with the axolemma (arrow). Magnification bar: 500 nm. (G) Progressive enlargement of presynaptic nerve terminals is dominated by delicate tubulovesicular elements. Magnification bar: 500 nm. (H) Typical dystrophic axon (arrow) is large with compact tubulovesicular elements. Magnification bar: 2 μm.
Figure 5
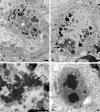
Accumulation of autophagosomes in presynaptic axonal terminals in aged SMG-CG. (A, B) Presynaptic nerve terminal with scattered autophagosomes with collections of synaptic vesicles (arrow, B), common in early dystrophic lesions. Magnification bars: A, 500 nm; B, 100 nm. (C) Dystrophic swellings surround occasional perikarya and typically contain the same aggregates of organelles, suggesting a possible spray of terminals originating from the same parent axon. Magnification bar: 2 μm.
Figure 6
Amorphous granular debris in presynaptic nerve terminals of aged mouse SMG-CG. (A) Presynaptic axonal terminal containing assorted subcellular organelles and small amounts of granular osmiophilic debris (arrow). (B, C) Marked swelling, no longer with synaptic specialization visible in this cross section contains multivesicular bodies and large aggregates of granular osmiophilic debris (arrows, B, C), seen better at higher magnification in C. (D) Dystrophic axon with large collections of osmiophilic material admixed with islands of tubulovesicular elements. Magnification bars: A–D, 500 nm.
Figure 7
Dendritic alterations in aged mouse SMG-CG. (A, B) The neuropil contains an enlarged dendrite with numerous lucent small tubulovesicular elements seen at higher magnification in B. Magnification bars: A, 2 μm; B, 500 nm. (C, D) Mitochondriopathy is represented by collections of large numbers of mitochondria smaller than those of adjacent cell body (arrow, C) and megamitochondria (arrow, D). Magnification bars: C, D, 2 μm.
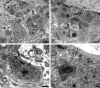
Figure 8
Mitochondriopathy in the perikarya of sympathetic neurons of aged mouse SMG-CG. (A) Enlarged mitochondria involving the perikaryon and adjacent proximal dendrites. Magnification bar: A, 10 μm. (B, C) Variation in the appearance of megamitochondria includes enlarged forms with relatively well maintained cristae (B), shortened cristae with lucent or granular matrix (C) or intramitochondrial vacuoles. Magnification bars: B, C, 2 μm.
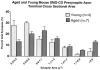
Figure 9
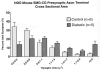
Diabetic and age-matched control mouse SMG-CG presynaptic axon terminal cross sectional area size-frequency histograms. (A) NOD mouse SMG-CG presynaptic axon terminal cross sectional area. (B) NOD mouse SCG presynaptic axon terminal cross sectional area.
Figure 9
Diabetic and age-matched control mouse SMG-CG presynaptic axon terminal cross sectional area size-frequency histograms. (A) NOD mouse SMG-CG presynaptic axon terminal cross sectional area. (B) NOD mouse SCG presynaptic axon terminal cross sectional area.
Figure 10
Accumulation of delicate tubulovesicular elements in presynaptic axon terminals of diabetic mouse SMG-CG. (A, B) Delicate tubulovesicular elements are intimately associated with active zones and adjacent axon terminal boutons. Magnification bar: A, B, 500 nm.
Figure 11
Accumulation of autophagosomes in diabetic presynaptic dystrophic axon terminals. (A, B) Presynaptic nerve terminal contents range from single multivesicular bodies (MVBs) (arrow, A) to large forms with numerous MVBs admixed with mitochondria (B). Magnification bars: A, 500 nm; B, 500 nm. (C, D) MVBs consist of collections of synaptic vesicles (arrow, C), common in early dystrophic lesions, to tubulovesicular elements in later dystrophic terminals (arrows, D). Magnification bars: C, 500 nm; D, 500 nm.
Figure 12
Mitochondriopathy in diabetic mouse SMG-CG. (A, B) Accumulation of mitochondria in a small process (arrow, A) within the perineuronal satellite cell sheath is postsynaptic to an enlarged axonal terminus (arrow, B). Magnification bars: A, 2 μm; B, 500 nm. (C, D) A markedly enlarged dendrite (arrow, C) whose synaptic specializations are not identified at this level, distorts the contours of the adjacent principal sympathetic neuron, and contains large numbers of mitochondria (arrow, D) that are considerably smaller and hyperchromatic than mitochondria in adjacent perikarya (arrowheads, D). Magnification bars: C, 2 μm; D, 500 nm. (E) Large numbers of mitochondria-laden dendritic processes may be particularly numerous in diabetic mouse SMG-CG. Magnification bar: 2 μm.
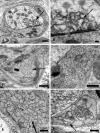
Figure 13. Dendritic tubulovesicular processes in diabetic mouse SMG-CG
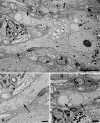
(A) The neuropil of this diabetic mouse ganglion contains both markedly enlarged neurites as well as numerous lucent small dendritic processes (arrows). Magnification bar: 10 μm. (B) An enlarged neurite within a satellite cell sheath results in the distortion of the contours of the adjacent neuronal cell body. Magnification bar: 2 μm. (C, D) A markedly dilated tubulovesicular-laden process shows a maintained presynaptic axon at its lower margin (arrow, C) seen at higher magnification in D. Magnification bars: C, 2 μm; D, 500 nm. (E) A small dendrite with a maintained synapse shows accumulated lucent tubulovesicular processes. Magnification bar: 500 nm. (F–H) The accumulated tubulovesicular elements are heterogeneous with lucent tubules and vesicles interspersed with cytoplasm (F), complex collections of coated vesicles forming unusual shapes (arrow, G), others part of multivesicular bodies (arrowhead, G), or delicate anastomosing tubulovesicular elements with proteinaceous intraluminal content (arrow, H). Magnification bars: F, 500 nm; G, 100 nm; H, 500 nm. (I, J) Occasional tubulovesicular elements appear to arise from the plasmalemma with partial clathrin coats (arrow, I) or contain residual ribosomes that may derive from rough endoplasmic reticulum (arrows, J). Magnification bars: I, 100 nm; J, 500 nm.
Figure 13. Dendritic tubulovesicular processes in diabetic mouse SMG-CG
(A) The neuropil of this diabetic mouse ganglion contains both markedly enlarged neurites as well as numerous lucent small dendritic processes (arrows). Magnification bar: 10 μm. (B) An enlarged neurite within a satellite cell sheath results in the distortion of the contours of the adjacent neuronal cell body. Magnification bar: 2 μm. (C, D) A markedly dilated tubulovesicular-laden process shows a maintained presynaptic axon at its lower margin (arrow, C) seen at higher magnification in D. Magnification bars: C, 2 μm; D, 500 nm. (E) A small dendrite with a maintained synapse shows accumulated lucent tubulovesicular processes. Magnification bar: 500 nm. (F–H) The accumulated tubulovesicular elements are heterogeneous with lucent tubules and vesicles interspersed with cytoplasm (F), complex collections of coated vesicles forming unusual shapes (arrow, G), others part of multivesicular bodies (arrowhead, G), or delicate anastomosing tubulovesicular elements with proteinaceous intraluminal content (arrow, H). Magnification bars: F, 500 nm; G, 100 nm; H, 500 nm. (I, J) Occasional tubulovesicular elements appear to arise from the plasmalemma with partial clathrin coats (arrow, I) or contain residual ribosomes that may derive from rough endoplasmic reticulum (arrows, J). Magnification bars: I, 100 nm; J, 500 nm.
Similar articles
- Neuritic dystrophy and neuronopathy in Akita (Ins2(Akita)) diabetic mouse sympathetic ganglia.
Schmidt RE, Green KG, Snipes LL, Feng D. Schmidt RE, et al. Exp Neurol. 2009 Mar;216(1):207-18. doi: 10.1016/j.expneurol.2008.11.019. Epub 2008 Dec 10. Exp Neurol. 2009. PMID: 19111542 Free PMC article. - Effect of insulin and an erythropoietin-derived peptide (ARA290) on established neuritic dystrophy and neuronopathy in Akita (Ins2 Akita) diabetic mouse sympathetic ganglia.
Schmidt RE, Feng D, Wang Q, Green KG, Snipes LL, Yamin M, Brines M. Schmidt RE, et al. Exp Neurol. 2011 Dec;232(2):126-35. doi: 10.1016/j.expneurol.2011.05.025. Epub 2011 Aug 18. Exp Neurol. 2011. PMID: 21872588 Free PMC article. - Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.
Salisbury L, Baraitser L. Salisbury L, et al. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review. - Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.
Lee CC, Chen CW, Yen HK, Lin YP, Lai CY, Wang JL, Groot OQ, Janssen SJ, Schwab JH, Hsu FM, Lin WH. Lee CC, et al. Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924 - Trends in Surgical and Nonsurgical Aesthetic Procedures: A 14-Year Analysis of the International Society of Aesthetic Plastic Surgery-ISAPS.
Triana L, Palacios Huatuco RM, Campilgio G, Liscano E. Triana L, et al. Aesthetic Plast Surg. 2024 Oct;48(20):4217-4227. doi: 10.1007/s00266-024-04260-2. Epub 2024 Aug 5. Aesthetic Plast Surg. 2024. PMID: 39103642 Review.
Cited by
- Temporal dystrophic remodeling within the intrinsic cardiac nervous system of the streptozotocin-induced diabetic rat model.
Menard CE, Durston M, Zherebitskaya E, Smith DR, Freed D, Glazner GW, Tian G, Fernyhough P, Arora RC. Menard CE, et al. Acta Neuropathol Commun. 2014 Jun 4;2:60. doi: 10.1186/2051-5960-2-60. Acta Neuropathol Commun. 2014. PMID: 24894521 Free PMC article. - Mitochondrial Perturbation in Alzheimer's Disease and Diabetes.
Akhter F, Chen D, Yan SF, Yan SS. Akhter F, et al. Prog Mol Biol Transl Sci. 2017;146:341-361. doi: 10.1016/bs.pmbts.2016.12.019. Epub 2017 Feb 4. Prog Mol Biol Transl Sci. 2017. PMID: 28253990 Free PMC article. Review. - Machine learning approaches reveal subtle differences in breathing and sleep fragmentation in _Phox2b_-derived astrocytes ablated mice.
Silva TM, Borniger JC, Alves MJ, Alzate Correa D, Zhao J, Fadda P, Toland AE, Takakura AC, Moreira TS, Czeisler CM, Otero JJ. Silva TM, et al. J Neurophysiol. 2021 Apr 1;125(4):1164-1179. doi: 10.1152/jn.00155.2020. Epub 2021 Jan 27. J Neurophysiol. 2021. PMID: 33502943 Free PMC article. - Mitochondrial stress and the pathogenesis of diabetic neuropathy.
Fernyhough P, Roy Chowdhury SK, Schmidt RE. Fernyhough P, et al. Expert Rev Endocrinol Metab. 2010 Jan 1;5(1):39-49. doi: 10.1586/eem.09.55. Expert Rev Endocrinol Metab. 2010. PMID: 20729997 Free PMC article.
References
- Dyck PJ, Jedrzejowska H, Karnes J, et al. Reconstruction of motor, sensory, and autonomic neurons based on morphometric study of sampled levels. Muscle Nerve. 1979;2:399–405. - PubMed
- Jarvi R, Helen P, Pelto-Huikko M, et al. Age-related changes of enkephalinergic innervation of human sympathetic neurons. Mech Ageing Dev. 1988;44:143–51. - PubMed
- Duchen LW, Anjorin A, Watkins PJ, et al. Pathology of autonomic neuropathy in diabetes mellitus. Ann Intern Med. 1980;92:301–3. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 DK019645/DK/NIDDK NIH HHS/United States
- AG10299/AG/NIA NIH HHS/United States
- R37 DK019645/DK/NIDDK NIH HHS/United States
- R01 AG010299/AG/NIA NIH HHS/United States
- R37 DK19645/DK/NIDDK NIH HHS/United States
- R01 DK019645-29/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical