Induction of T regulatory cells attenuates idiopathic nephrotic syndrome - PubMed (original) (raw)
Induction of T regulatory cells attenuates idiopathic nephrotic syndrome
Ludmilla Le Berre et al. J Am Soc Nephrol. 2009 Jan.
Abstract
Buffalo/Mna rats spontaneously develop FSGS and nephrotic syndrome as a result of an immune disorder. Similar to some humans with FSGS, the disease recurs after renal transplantation, suggesting the involvement of a circulating factor. Here, we tested the effect of several immunosuppressive treatments on these rats. Although corticosteroids, cyclosporin A, and anti-T cell receptor treatment reduced proteinuria, only the deoxyspergualin derivative LF15-0195 led to a rapid and complete normalization of proteinuria. Furthermore, this compound led to the regression of renal lesions during both the initial disease and posttransplantation recurrence. The frequency of splenic and peripheral CD4+CD25+FoxP3+ T lymphocytes significantly increased with remission. Moreover, the transfer of purified LF15-0195-induced CD4+CD25+ T cells to irradiated Buff/Mna rats significantly reduced their proteinuria compared with the transfer of untreated control cells, suggesting that LF15-0195 induces regulatory T cells that are able to induce regression of rat nephropathy. These data suggest that idiopathic nephrotic syndrome/FSGS disease can be regulated by cellular transfer, but how this regulation leads to the reorganization of the podocyte cytoskeleton remains to be determined.
Figures
Figure 1.
(A) Beneficial effect of a derivative of DSG, LF15-0195, on both the onset of proteinuria and established proteinuria in young Buff/Mna rats. Each treatment was administered for 30 d at a dosage of 1 mg/kg per d (n = 5). The control group consisted of vehicle-treated Buff/Mna rats. (B) Effect of LF15-0195 compared with LF15-0296, an isomer without any immunosuppressive effect, on the proteinuria of 6-mo-old Buff/Mna rats. The two treatments were administered for 30 d (n = 5). (C) LF15-0195 reduced the proteinuria of Buff/Mna rats experiencing recurrence of their initial disease after kidney transplantation. The treatment was administered for 30 d (n = 5). The control group consisted of untreated Buff/Mna rats experiencing recurrence of proteinuria after kidney transplantation (n = 5). Proteinuria is expressed as the ratio of the urinary proteins (g/L) to urinary creatinine concentration (mmol/L). Data are means ± SEM (scale bar). *Significant decrease in proteinuria (versus day 0), P < 0.05; **P < 0.01.
Figure 2.
(A through D) Light microscopic examination of native kidneys from untreated age-matched Buff/Mna rats showing major tubular alterations (A) and FSGS lesions (C; n = 4) and LF15-0195-treated Buff/Mna kidneys showing a subnormal renal interstitium (B) without glomerulosclerosis (D; n = 6; periodic acid-Schiff staining). (E and F) Electron microscopic examination of age-matched kidneys of native Buff/Mna rats showing some flattening and foot process fusion (E), compared with LF15-0195–treated Buff/Mna kidneys showing a normal ultrastructure with well differentiated and organized foot processes (F). Magnifications: ×100 in A and B; ×200 in C and D; ×15,000 in F.
Figure 3.
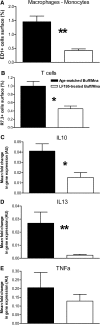
Decrease in renal infiltration of monocytes, T lymphocytes, and associated cytokines upon LF15-0195 treatment. (A and B) Results are expressed as the percentage (means ± SEM) of the surface area occupied by positive cells. Each group consisted of five or six rats. The control groups consisted of age-matched Buff/Mna rats. (A) Monocyte-macrophage (ED1 Ab) populations. (B) T lymphocytes (R7.3 Ab). (C through E) IL-10 (C), IL-13 (D), and TNF-α (E) mRNA expression was analyzed by quantitative RT-PCR in the kidneys of LF15-0195–treated or untreated age-matched Buff/Mna rats. Data are means ± SEM of arbitrary units (AU) relative to HPRT (n = 6). *P < 0.05; **P < 0.01.
Figure 4.
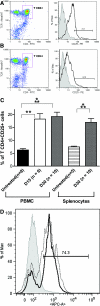
Significant increase in blood CD4+CD25+ T lymphocytes after LF15-0195 treatment of Buff/Mna rats. (A and B) Representative FACS staining of PBMC of LF15-0195–treated Buff/Mna (A) versus untreated age-matched Buff/Mna rats (B) shows a significant increase in the frequency of CD4+CD25+ T cells (similar dot plots and histograms not shown for splenocytes). (C) Results are expressed as the percentage of CD4+CD25+ T cells in PBMC or splenocytes of untreated or treated Buff/Mna rats at either day 15 or the end (day 30) of the treatment. (A through C) Cells labeled with control isotype antibodies were used as a negative control and are represented in gray on the histogram. (D) Representative histogram of the percentage of FoxP3-positive cells (FoxP3-PE) contained in the CD4+CD25+ T cells as described previously for LF15-0195–treated rats. The dotted line in the histogram represents the percentage of FoxP3+ cells in CD4+CD25+ T cells from untreated rats. *P < 0.05; **P < 0.01.
Figure 5.
Accumulation of transcripts for the regulatory markers CD25, CTLA4, GITR, IDO, and iNOS in LF15-0195–treated in comparison with untreated age-matched spleens. mRNA expression was analyzed by quantitative RT-PCR. Data are means ± SEM of AU relative to HPRT (n = 6). *P < 0.05; **P < 0.01; ***P < 0.001.
Figure 6.
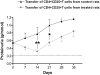
Transient regulation of nephropathy after transfer of CD4+CD25+ T cells from Buff/Mna rats in remission into Buff/Mna rats. Purified CD4+CD25+ T cells from LF15-0195–treated Buff/Mna were injected into 4 Gy–irradiated proteinuric Buff/Mna rats (n = 5). The number of injected cells was equivalent in the two groups: 2.57 ± 0.8 × 106 cells in the untreated group and 2.97 ± 0.8 × 106 cells in the LF15-0195 treated group (NS). Data are means ± SEM (scale bar) and are compared with a control group injected with purified cells from untreated rats (n = 5). *P < 0.05, **P < 0.01 between the two experimental groups.
Similar articles
- Is there B cell involvement in a rat model of spontaneous idiopathic nephrotic syndrome treated with LF15-0195?
Le Berre L, Tilly G, Dantal J. Le Berre L, et al. J Nephrol. 2014 Jun;27(3):265-73. doi: 10.1007/s40620-014-0081-0. Epub 2014 Mar 25. J Nephrol. 2014. PMID: 24664644 - The Immunosuppressive Drug LF15-0195 Acts Also on Glomerular Lesions, by a Change in Cytoskeleton Distribution in Podocyte.
Le Berre L, Tilly G, Pilet P, Brouard S, Dantal J. Le Berre L, et al. Am J Nephrol. 2024;55(5):583-596. doi: 10.1159/000539965. Epub 2024 Jul 29. Am J Nephrol. 2024. PMID: 39074452 - Renal macrophage activation and Th2 polarization precedes the development of nephrotic syndrome in Buffalo/Mna rats.
Le Berre L, Hervé C, Buzelin F, Usal C, Soulillou JP, Dantal J. Le Berre L, et al. Kidney Int. 2005 Nov;68(5):2079-90. doi: 10.1111/j.1523-1755.2005.00664.x. Kidney Int. 2005. PMID: 16221207 - Circulating permeability factors in idiopathic nephrotic syndrome and focal segmental glomerulosclerosis.
McCarthy ET, Sharma M, Savin VJ. McCarthy ET, et al. Clin J Am Soc Nephrol. 2010 Nov;5(11):2115-21. doi: 10.2215/CJN.03800609. Epub 2010 Oct 21. Clin J Am Soc Nephrol. 2010. PMID: 20966123 Review. - The treatment of idiopathic focal segmental glomerulosclerosis in adults.
Hogan J, Radhakrishnan J. Hogan J, et al. Adv Chronic Kidney Dis. 2014 Sep;21(5):434-41. doi: 10.1053/j.ackd.2014.03.016. Adv Chronic Kidney Dis. 2014. PMID: 25168833 Review.
Cited by
- Characterizing Glomerular Barrier Dysfunction with Patient-Derived Serum in Glomerulus-on-a-Chip Models: Unveiling New Insights into Glomerulonephritis.
Kim SY, Choi YY, Kwon EJ, Seo S, Kim WY, Park SH, Park S, Chin HJ, Na KY, Kim S. Kim SY, et al. Int J Mol Sci. 2024 May 8;25(10):5121. doi: 10.3390/ijms25105121. Int J Mol Sci. 2024. PMID: 38791159 Free PMC article. - Immunosuppressive agents for frequently relapsing/steroid-dependent nephrotic syndrome in children: a systematic review and network meta-analysis.
Zhu Y, Chen J, Zhang Y, Wang X, Wang J. Zhu Y, et al. Front Immunol. 2024 Feb 23;15:1310032. doi: 10.3389/fimmu.2024.1310032. eCollection 2024. Front Immunol. 2024. PMID: 38464533 Free PMC article. - Rituximab in the treatment of primary FSGS: time for its use in routine clinical practice?
Morris AD, Floyd L, Woywodt A, Dhaygude A. Morris AD, et al. Clin Kidney J. 2023 May 24;16(8):1199-1205. doi: 10.1093/ckj/sfad122. eCollection 2023 Aug. Clin Kidney J. 2023. PMID: 37529639 Free PMC article. - State of the art in childhood nephrotic syndrome: concrete discoveries and unmet needs.
Vincenti F, Angeletti A, Ghiggeri GM. Vincenti F, et al. Front Immunol. 2023 Jul 12;14:1167741. doi: 10.3389/fimmu.2023.1167741. eCollection 2023. Front Immunol. 2023. PMID: 37503337 Free PMC article. Review. - CD8 T Cell-Derived Exosomal miR-186-5p Elicits Renal Inflammation via Activating Tubular TLR7/8 Signal Axis.
Xu X, Qu S, Zhang C, Zhang M, Qin W, Ren G, Bao H, Li L, Zen K, Liu Z. Xu X, et al. Adv Sci (Weinh). 2023 Sep;10(25):e2301492. doi: 10.1002/advs.202301492. Epub 2023 Jul 3. Adv Sci (Weinh). 2023. PMID: 37395441 Free PMC article.
References
- Dantal J, Giral M, Hourmant M, Soulillou JP: Glomerulonephritis recurrences after kidney transplantation. Curr Opin Nephrol Hypertens 4: 146–154, 1995 - PubMed
- Artero ML, Sharma R, Savin VJ, Vincenti F: Plasmapheresis reduces proteinuria and serum capacity to injure glomeruli in patients with recurrent focal glomerulosclerosis. Am J Kidney Dis 23: 574–581, 1994 - PubMed
- Dantal J, Bigot E, Bogers W, Testa A, Kriaa F, Jacques Y, Hurault de Ligny B, Niaudet P, Charpentier B, Soulillou JP: Effect of plasma protein adsorption on protein excretion in kidney-transplant recipients with recurrent nephrotic syndrome. N Engl J Med 330: 7–14, 1994 - PubMed
- Dantal J, Godfrin Y, Koll R, Perretto S, Naulet J, Bouhours JF, Soulillou JP: Antihuman immunoglobulin affinity immunoadsorption strongly decreases proteinuria in patients with relapsing nephrotic syndrome. J Am Soc Nephrol 9: 1709–1715, 1998 - PubMed
- Oliver JD 3rd, Simons JL, Troy JL, Provoost AP, Brenner BM, Deen WM: Proteinuria and impaired glomerular permselectivity in uninephrectomized fawn-hooded rats. Am J Physiol 267: F917–F25, 1994 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials