Innate immune response to viral infection of the lungs - PubMed (original) (raw)
Review
Innate immune response to viral infection of the lungs
Hayley See et al. Paediatr Respir Rev. 2008 Dec.
Abstract
Viral respiratory tract infections are the most common infectious illnesses, though they are usually self-limiting and confined to the respiratory tract. The rapid identification of viruses and their effective elimination with minimal local and systemic inflammation is a testament to the efficiency of the innate immune response within the airways and lungs. A failure of this response appears to occur in those with asthma and chronic obstructive pulmonary disease, where viral infection is an important trigger for acute exacerbations. The innate immune response to viruses requires their early detection through pathogen recognition receptors and the recruitment of the efficient antiviral response that is centred around the release of type 1 interferons. The airway epithelium provides both a barrier and an early detector for viruses, and interacts closely with cells of the innate immune response, especially macrophages and dendritic cells, to eliminate infection and trigger a specific adaptive immune response.
Figures
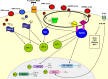
Figure 1
RNA helicases, retinoic-acid-inducible protein (RIG)-I and melanoma-differentiation-associated gene (MDA)-5 detect viral replication in the cytosol. RIG-I detects negative-sense RNA from DNA viruses and MDA-5 detects positive-sense RNA from RNA viruses. RIG-I and MDA-5 signal through interferon response factor (IRF)-3 and IRF-7 to induce interferon (IFN)-β and NF-κB, leading to the transcription of pro-inflammatory mediators. TLR-3 is expressed in intracellular endosomes and responds to the presence of double-stranded RNA (dsRNA) which forms as a product of the replication of the majority of RNA viruses. TLR-3 is dependent on binding with the Toll/IL-1 receptor domain-containing adaptor (TRIF). This leads to phosphorylation of IRF-3 which forms a homodimer and translocates to the nucleus, resulting in the expression of IFN-β. Signalling can also occur that leads to the translocation of NF-κB. TLR-4 is expressed on the surface of cells and recognizes bacterial endotoxin, lipopolysaccharide and the F protein of respiratory syncytial virus (RSV). TLR-4 is able to signal via both MyD88-dependent and -independent pathways and is able to activate a response via IRF-3, NF-κB and activating protein (AP)-1. TLR-7 is found within endosomes and is activated by single-stranded RNA (ssRNA). TLR-9 is also found within endosomes and is activated by unmethylated CpG dsRNA. Both TLR-7 and -9 signal through a MyD88-dependent pathway, leading to the translocation of NF-κB, AP-1 and IRF-7, and the latter is responsible for release of IFN-α. The IFNs mediate their effects through the induction of hundreds of IFN stimulated genes (ISGs), the actions of many of which remain unknown. Induction of IFN-β and IFN-α4 occurs early after virus infection and is regulated by phosphorylation of IRF-3. The other IFN-α genes require synthesis of IRF-7 to lead to their activation and this occurs as a more delayed response requiring a positive feedback signal via the early release of IFN-β/α4 to lead to the full induction of ISGs. Release of type 1 IFNs can be recognized by infected and neighbouring cells and type 1 IFNs exert their actions through specific receptors (IFN receptor-α1/α2). Receptor engagement leads to activation of the IFN stimulated regulatory factor (ISGF)-3 comprised of members of the signal transducers and activators of transcription (STAT)-1 and -2, as well as IRF-9. This complex can directly bind in the nucleus to the IFN stimulated response element, leading to transcription of type 1 IFNs as well as ISGs, especially the antiviral proteins: protein kinase receptor (PKR), RNAse-I and 2′,5′′′ oligoadenylate synthetase, and myxovirus resistance proteins (MxA).
Similar articles
- The host immune response in respiratory virus infection: balancing virus clearance and immunopathology.
Newton AH, Cardani A, Braciale TJ. Newton AH, et al. Semin Immunopathol. 2016 Jul;38(4):471-82. doi: 10.1007/s00281-016-0558-0. Epub 2016 Mar 10. Semin Immunopathol. 2016. PMID: 26965109 Free PMC article. Review. - Innate immune mechanism in allergic asthma.
Suarez CJ, Parker NJ, Finn PW. Suarez CJ, et al. Curr Allergy Asthma Rep. 2008 Sep;8(5):451-9. doi: 10.1007/s11882-008-0085-8. Curr Allergy Asthma Rep. 2008. PMID: 18682113 Review. - Susceptibility to viral infections in chronic obstructive pulmonary disease: role of epithelial cells.
Sajjan US. Sajjan US. Curr Opin Pulm Med. 2013 Mar;19(2):125-32. doi: 10.1097/MCP.0b013e32835cef10. Curr Opin Pulm Med. 2013. PMID: 23361194 Review. - Innate immunity and chronic obstructive pulmonary disease: a mini-review.
Shaykhiev R, Crystal RG. Shaykhiev R, et al. Gerontology. 2013;59(6):481-9. doi: 10.1159/000354173. Epub 2013 Sep 3. Gerontology. 2013. PMID: 24008598 Free PMC article. Review. - Deciphering Respiratory-Virus-Associated Interferon Signaling in COPD Airway Epithelium.
Guo-Parke H, Linden D, Weldon S, Kidney JC, Taggart CC. Guo-Parke H, et al. Medicina (Kaunas). 2022 Jan 13;58(1):121. doi: 10.3390/medicina58010121. Medicina (Kaunas). 2022. PMID: 35056429 Free PMC article. Review.
Cited by
- Applications of the phytomedicine Echinacea purpurea (Purple Coneflower) in infectious diseases.
Hudson JB. Hudson JB. J Biomed Biotechnol. 2012;2012:769896. doi: 10.1155/2012/769896. Epub 2011 Oct 26. J Biomed Biotechnol. 2012. PMID: 22131823 Free PMC article. Review. - Recombinant human elafin protects airway epithelium integrity during inflammation.
Li Q, Zhou XD, Xu XY, Yang J. Li Q, et al. Mol Biol Rep. 2010 Jul;37(6):2981-8. doi: 10.1007/s11033-009-9865-z. Epub 2009 Oct 13. Mol Biol Rep. 2010. PMID: 19823954 - The airway epithelium: soldier in the fight against respiratory viruses.
Vareille M, Kieninger E, Edwards MR, Regamey N. Vareille M, et al. Clin Microbiol Rev. 2011 Jan;24(1):210-29. doi: 10.1128/CMR.00014-10. Clin Microbiol Rev. 2011. PMID: 21233513 Free PMC article. Review. - Qiangzhi decoction protects mice from influenza A pneumonia through inhibition of inflammatory cytokine storm.
Zhu HY, Huang H, Shi XL, Zhou W, Zhou P, Yan QL, Zhu HG, Ju DW. Zhu HY, et al. Chin J Integr Med. 2015 May;21(5):376-83. doi: 10.1007/s11655-014-2020-2. Epub 2014 Dec 17. Chin J Integr Med. 2015. PMID: 25519444 Free PMC article. - A predominately pulmonary activation of complement in a mouse model of severe COVID-19.
Szachowicz PJ, Wohlford-Lenane C, Heinen CJ, Ghimire S, Xue B, Boly TJ, Verma A, MašinoviĆ L, Bermick JR, Perlman S, Meyerholz DK, Pezzulo AA, Zhang Y, Smith RJH, McCray PB Jr. Szachowicz PJ, et al. bioRxiv [Preprint]. 2024 Jun 3:2024.05.31.596892. doi: 10.1101/2024.05.31.596892. bioRxiv. 2024. PMID: 38895461 Free PMC article. Preprint.
References
- Manoha C., Espinosa S., Aho S., Huet F., Pothier P. Epidemiological and clinical features of hMPV, RSV and RVs infections in young children. J Clin Virol. 2007;38:221–226. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical