Passive immunotherapy rapidly increases structural plasticity in a mouse model of Alzheimer disease - PubMed (original) (raw)
Passive immunotherapy rapidly increases structural plasticity in a mouse model of Alzheimer disease
Tara L Spires-Jones et al. Neurobiol Dis. 2009 Feb.
Abstract
Senile plaque-associated changes in neuronal connectivity such as altered neurite trajectory, dystrophic swellings, and synapse and dendritic spine loss are thought to contribute to cognitive dysfunction in Alzheimer's disease and mouse models. Immunotherapy to remove amyloid beta is a promising therapy that causes recovery of neurite trajectory and dystrophic neurites over a period of days. The acute effects of immunotherapy on neurite morphology at a time point when soluble amyloid has been cleared but dense plaques are not yet affected are unknown. To examine whether removal of soluble amyloid beta (Abeta) has a therapeutic effect on dendritic spines, we explored spine dynamics within 1 h of applying a neutralizing anti Abeta antibody. This acute treatment caused a small but significant increase in dendritic spine formation in PDAPP brain far from plaques, without affecting spine plasticity near plaques or average dendritic spine density. These data support the hypothesis that removing toxic soluble forms of amyloid-beta rapidly increases structural plasticity possibly allowing functional recovery of neural circuits.
Figures
Figure 1
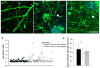
PDAPP animals exhibit dendritic curvature and dendritic spine loss near plaques. Before antibody treatment, spiny dendrites (green) were observed in control (a) and PDAPP animals (b,c). Near plaques (blue), spiny dendrites were significantly curvier than those farther from plaques and curvier than dendrites in control animals (Mann-Whitney test control vs PDAPP and PDAPP <50 μm from plaque vs >50 μm from plaque, both p<0.05). Curvature ratios of individual spiny dendrite segments are plotted in (d). The arrow in (b) shows a particularly curved dendrite near a plaque. Dendritic spines are also lost near plaques in PDAPP mice (e * p<0.01 ANOVA). The arrowhead in (c) points to a dendrite that becomes less spiny as it approaches the plaque. Scale bar 10 μm.
Figure 2
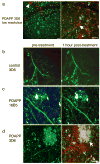
Treatment with AlexaFlour 594 conjugated 3D6 labels amyloid pathology in PDAPP cortex. Low resolution images of PDAPP brain treated with labeled 3D6 (a) show cerebral amyloid angiopathy (arrowhead) dense plaques (large arrows), and diffuse amyloid (small arrows) are labeled red after treatment. No labeling was observed in either non-transgenic control animals treated with labeled 3D6 (b) or in PDAPP animals treated with labeled 16B5, an antibody to human tau which should not be present in mouse brain (c). High resolution images of PDAPP brain treated with AlexaFlour 594 conjugated 3D6 shows that plaques are labeled 1 hour after treatment but not before antibody is applied (d). Neurites are filled with dextrans (green) and dense plaques are labeled with methoxy XO4 (blue). Scale bars 50 μm (a), 10 μm (b–d)
Figure 3
Structural plasticity increases with antibody treatment. Individual dendritic spines (n=758) were followed before treatment and one hour after antibody application. New spines were occasionally observed after one hour (arrow, a), and some spines were eliminated within one hour (arrow, b). Reconstruction of dendrites (blue) and spine heads (green) highlights the formation and elimination. The percent formation and elimination for each dendritic segment were compared to the upper quartile for non-transgenic % formation and elimination using contingency table analysis. With this analysis, we observe that significantly more spines are formed distant from plaques in PDAPP animals one hour after 3D6 treatment compared to control animals (Chi squared p=0.0177, c). Thus structural plasticity increases rapidly following anti Aβ antibody treatment but not control antibody treatment. Scale bar 2 μm.
Figure 4
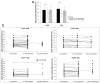
The average number of dendritic spines per micron of spiny dendrite length for each group (control dendrite segments, PDAPP dendrite segments within 50 μm of a plaque, and PDAPP dendrites far from plaques) was unchanged one hour after antibody treatment (a). Following individual dendritic segments over one hour and in some cases 1 day (b) shows no great changes in spine density over these time periods as would be expected since spine formation and elimination are very rare events. * p < 0.05 post-hoc one way ANOVA.
Figure 5
Axonal curvature in PDAPP mice near plaques does not recover within 1 day of antibody treatment. Axonal neurofilament was stained with smi312 (red) to measure axon curvature near and far from ThioS labeled plaques (blue). Arrows show curvy dendrites and the arrowhead shows smi312 positive plaque-associated dystrophies persist for 1 day after 3D6 treatment. Scale bar 20 μm.
Similar articles
- Spines, plasticity, and cognition in Alzheimer's model mice.
Spires-Jones T, Knafo S. Spires-Jones T, et al. Neural Plast. 2012;2012:319836. doi: 10.1155/2012/319836. Epub 2011 Nov 28. Neural Plast. 2012. PMID: 22203915 Free PMC article. Review. - A single dose of passive immunotherapy has extended benefits on synapses and neurites in an Alzheimer's disease mouse model.
Rozkalne A, Spires-Jones TL, Stern EA, Hyman BT. Rozkalne A, et al. Brain Res. 2009 Jul 14;1280:178-85. doi: 10.1016/j.brainres.2009.05.045. Epub 2009 May 22. Brain Res. 2009. PMID: 19465012 Free PMC article. - Dendritic spine abnormalities in amyloid precursor protein transgenic mice demonstrated by gene transfer and intravital multiphoton microscopy.
Spires TL, Meyer-Luehmann M, Stern EA, McLean PJ, Skoch J, Nguyen PT, Bacskai BJ, Hyman BT. Spires TL, et al. J Neurosci. 2005 Aug 3;25(31):7278-87. doi: 10.1523/JNEUROSCI.1879-05.2005. J Neurosci. 2005. PMID: 16079410 Free PMC article. - Dendritic spine density, morphology, and fibrillar actin content surrounding amyloid-β plaques in a mouse model of amyloid-β deposition.
Kirkwood CM, Ciuchta J, Ikonomovic MD, Fish KN, Abrahamson EE, Murray PS, Klunk WE, Sweet RA. Kirkwood CM, et al. J Neuropathol Exp Neurol. 2013 Aug;72(8):791-800. doi: 10.1097/NEN.0b013e31829ecc89. J Neuropathol Exp Neurol. 2013. PMID: 23860033 Free PMC article. - Neuronal structure is altered by amyloid plaques.
Spires TL, Hyman BT. Spires TL, et al. Rev Neurosci. 2004;15(4):267-78. doi: 10.1515/revneuro.2004.15.4.267. Rev Neurosci. 2004. PMID: 15526551 Review.
Cited by
- Antibody-conjugated, dual-modal, near-infrared fluorescent iron oxide nanoparticles for antiamyloidgenic activity and specific detection of amyloid-β fibrils.
Skaat H, Corem-Slakmon E, Grinberg I, Last D, Goez D, Mardor Y, Margel S. Skaat H, et al. Int J Nanomedicine. 2013;8:4063-76. doi: 10.2147/IJN.S52833. Epub 2013 Oct 29. Int J Nanomedicine. 2013. PMID: 24194640 Free PMC article. - Novel amyloid-beta specific scFv and VH antibody fragments from human and mouse phage display antibody libraries.
Medecigo M, Manoutcharian K, Vasilevko V, Govezensky T, Munguia ME, Becerril B, Luz-Madrigal A, Vaca L, Cribbs DH, Gevorkian G. Medecigo M, et al. J Neuroimmunol. 2010 Jun;223(1-2):104-14. doi: 10.1016/j.jneuroim.2010.03.023. Epub 2010 May 6. J Neuroimmunol. 2010. PMID: 20451261 Free PMC article. - Spines, plasticity, and cognition in Alzheimer's model mice.
Spires-Jones T, Knafo S. Spires-Jones T, et al. Neural Plast. 2012;2012:319836. doi: 10.1155/2012/319836. Epub 2011 Nov 28. Neural Plast. 2012. PMID: 22203915 Free PMC article. Review. - The development of ADAM10 endocytosis inhibitors for the treatment of Alzheimer's disease.
Musardo S, Therin S, Pelucchi S, D'Andrea L, Stringhi R, Ribeiro A, Manca A, Balducci C, Pagano J, Sala C, Verpelli C, Grieco V, Edefonti V, Forloni G, Gardoni F, Meli G, Di Marino D, Di Luca M, Marcello E. Musardo S, et al. Mol Ther. 2022 Jul 6;30(7):2474-2490. doi: 10.1016/j.ymthe.2022.03.024. Epub 2022 Apr 4. Mol Ther. 2022. PMID: 35390543 Free PMC article. - Transient dynamics of Aβ contribute to toxicity in Alzheimer's disease.
Hubin E, van Nuland NA, Broersen K, Pauwels K. Hubin E, et al. Cell Mol Life Sci. 2014 Sep;71(18):3507-21. doi: 10.1007/s00018-014-1634-z. Epub 2014 May 7. Cell Mol Life Sci. 2014. PMID: 24803005 Free PMC article. Review.
References
- Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, Seubert P, Schenk D, Yednock T. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6(8):916–9. - PubMed
- Chen G, Chen KS, Knox J, Inglis J, Bernard A, Martin SJ, Justice A, McConlogue L, Games D, Freedman SB, Morris RG. A learning deficit related to age and beta-amyloid plaques in a mouse model of Alzheimer’s disease. Nature. 2000;408(6815):975–9. - PubMed
- Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci. 2005;8(1):79–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 AG062421/AG/NIA NIH HHS/United States
- R01 AG008487/AG/NIA NIH HHS/United States
- T32 AG000277/AG/NIA NIH HHS/United States
- R01 EB000768/EB/NIBIB NIH HHS/United States
- P50 AG005134/AG/NIA NIH HHS/United States
- AG00277/AG/NIA NIH HHS/United States
- EB00768/EB/NIBIB NIH HHS/United States
- R01 AG026249/AG/NIA NIH HHS/United States
- AG08487/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases