Host cell autophagy is induced by Toxoplasma gondii and contributes to parasite growth - PubMed (original) (raw)
Host cell autophagy is induced by Toxoplasma gondii and contributes to parasite growth
Yubao Wang et al. J Biol Chem. 2009.
Abstract
Autophagy has been shown to contribute to defense against intracellular bacteria and parasites. In comparison, the ability of such pathogens to manipulate host cell autophagy to their advantage has not been examined. Here we present evidence that infection by Toxoplasma gondii, an intracellular protozoan parasite, induces host cell autophagy in both HeLa cells and primary fibroblasts, via a mechanism dependent on host Atg5 but independent of host mammalian target of rapamycin suppression. Infection led to the conversion of LC3 to the autophagosome-associated form LC3-II, to the accumulation of LC3-containing vesicles near the parasitophorous vacuole, and to the relocalization toward the vacuole of structures labeled by the phosphatidylinositol 3-phosphate indicator YFP-2xFYVE. The autophagy regulator beclin 1 was concentrated in the vicinity of the parasitophorous vacuole in infected cells. Inhibitor studies indicated that parasite-induced autophagy is dependent on calcium signaling and on abscisic acid. At physiologically relevant amino acid levels, parasite growth became defective in Atg5-deficient cells, indicating a role for host cell autophagy in parasite recovery of host cell nutrients. A flow cytometric analysis of cell size as a function of parasite content revealed that autophagy-dependent parasite growth correlates with autophagy-dependent consumption of host cell mass that is dependent on parasite progression. These findings indicate a new role for autophagy as a pathway by which parasites may effectively compete with the host cell for limiting anabolic resources.
Figures
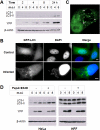
FIGURE 1.
Toxoplasma infection induces host cell autophagy. A, HeLa cells were infected with YFP-expressing T. gondii at the indicated multiplicity of infection (m.o.i) for 2, 4, 8, or 24 h. The cell lysates were subjected to immunoblotting analysis. B, HeLa cells were transfected with GFP-LC3 and then infected with wild-type T. gondii for 24 h. The cells were fixed and stained with DAPI prior to fluorescent imaging. Scale bars, 5 μm. C, cells were treated as in_B_. To detect signal both adjacent to and overlying the parasitophorous vacuole, a reconstruction was performed with confocal z-stack images. The arrows indicate parasitophorous vacuoles. D, HeLa or HFF cells were infected with YFP-expressing T. gondii for 22 h and then incubated with or without 10 μg/ml pepstatin A and 10 μg/ml E64D (PepA+E64D) for 2 h. The protein extracts were analyzed by immunoblotting using the indicated antibodies.
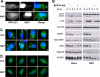
FIGURE 2.
Roles of autophagic pathway components in _T. gondii_-induced autophagy. A, control (WT) or_atg5_-/- MEFs were infected with YFP-expressing T. gondii for 22 h. The indicated samples were treated with pepstatin A (PepA) for 2 h prior to immunoblotting. B, HeLa cells were transfected with YFP-2×FYVE and infected with wild-type T. gondii for 24 h at a multiplicity of infection (m.o.i) of 4. The cells were fixed and stained with DAPI prior to fluorescent imaging. The_asterisk_ indicates a parasitophorous vacuole. Scale bar, 5 μm. C, HeLa cells were transfected with FLAG-Beclin 1 and infected with wild-type T. gondii for 24 h at a multiplicity of infection of 4. The cells were fixed and stained with DAPI prior to fluorescent imaging. Arrows indicate parasitophorous vacuoles. N, host nucleus. Scale bar, 5 μm. D, lysates of infected and control HeLa cells were subjected to immunoprecipitation (IP) using anti-Beclin 1. Input (20 μg) and IP samples were resolved by SDS-PAGE and probed with the indicated antibodies. E, HeLa cells were transfected with either non-specific (ns) or beclin 1 siRNA. After 2 days, the cells were infected with T. gondii for 24 h and analyzed by immunoblotting.
FIGURE 3.
_T. gondii_-induced autophagy is independent of mTOR. A, protein extracts from control or infected HeLa or HFF cells were analyzed with the indicated antibodies. B, wild-type or_tsc2_-/- MEFs were infected with YFP-expressing T. gondii for 24 h. The protein extracts were resolved by SDS-PAGE and probed with the indicated antibodies. For 4E-BP1, increased phosphorylation in_tsc2_-/- MEFs is reflected by the shift of intensity to the uppermost band. C, cells were infected as in B for 4 or 24 h, followed by trypsinization and fixation. Parasite proliferation was determined by flow cytometry as the number of parasites per infected cell.m.o.i, multiplicity of infection; Un, uninfected.
FIGURE 4.
_T. gondii_-induced autophagy is calcium-dependent. A, HeLa cells were transfected with GFP-LC3, infected with YFP-expressing T. gondii for 22 h, and then treated with either Me2SO vehicle (0.2%, v/v) or 20 μ
m
BAPTA-AM (BAP) for 2 h. The cells were fixed and stained with DAPI prior to fluorescent imaging, Scale bar, 5 μm. B, HeLa or HFF cells were infected and treated with vehicle or BAPTA-AM as in A. The immunoblots were probed with the indicated antibodies. C and_D_, HeLa cells (transfected with YFP-2×FYVE) were either infected with T. gondii for 22 h (C) or incubated in medium (D). The cells were then treated with vehicle (Con) or BAPTA-AM (BAP) for 2 h, followed by fixation, DAPI staining, and fluorescent imaging. The arrows indicate parasitophorous vacuoles. Scale bar, 5 μm. m.o.i, multiplicity of infection.
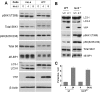
FIGURE 5.
Fluridone inhibits _T. gondii_-induced autophagy. HeLa cells were infected or not with YFP-expressing T. gondii for 4 h. After washing away free parasites, the cells were treated with vehicle (0.1% dimethyl sulfoxide (DMSO)) or 50 μ
m
fluridone in medium for 20 h. A, protein extracts were immunoblotted and probed with the indicated antibodies. The blot includes replicate samples from a single experiment. B, the intensity of the LC3-II band in immunoblots was measured by densitometry and normalized (untreated control = 100). Each_bar_ represents the mean value from two independent experiments.m.o.i, multiplicity of infection.
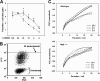
FIGURE 6.
Role of host cell autophagy in parasite growth. A, autophagy enhances growth at physiological amino acid levels. MEFs, either wild-type (filled circles) or Atg5_-/- (open circles), were deprived of serum for 24 h, infected with YFP-expressing_T. gondii for 4 h, rinsed to remove free parasites, and then incubated with the indicated dilutions of DMEM in Hanks buffer for 16 h. The cells were trypsinized, fixed, and analyzed by flow cytometry to measure parasite number/cell. This number was used to calculate parasite net gain by subtracting the values obtained at 4 h. The asterisks indicate_p_ values < 0.02. B and C, infection under limiting amino acids progressively reduces host cell size via autophagy. The cells analyzed in A were assessed for forward scatter (FSC) as a function of parasite content (YFP). B, dot plot displaying a representative sample of wt host cells in 100% DMEM after overnight infection.C, loess smoothing was applied to dot plot data for cells containing 1–20 parasites. Forward scatter values were normalized to the mean values of uninfected cells in each sample. Each curve represents data from the dot plot of one sample. For simplicity, only one representative sample (of three total) is displayed for cells cultured in 12, 25, or 50% DMEM. The data are representative of two similar experiments.
Similar articles
- Toxoplasma gondii-induced activation of EGFR prevents autophagy protein-mediated killing of the parasite.
Muniz-Feliciano L, Van Grol J, Portillo JA, Liew L, Liu B, Carlin CR, Carruthers VB, Matthews S, Subauste CS. Muniz-Feliciano L, et al. PLoS Pathog. 2013;9(12):e1003809. doi: 10.1371/journal.ppat.1003809. Epub 2013 Dec 19. PLoS Pathog. 2013. PMID: 24367261 Free PMC article. - ISG15 Connects Autophagy and IFN-γ-Dependent Control of Toxoplasma gondii Infection in Human Cells.
Bhushan J, Radke JB, Perng YC, Mcallaster M, Lenschow DJ, Virgin HW, Sibley LD. Bhushan J, et al. mBio. 2020 Oct 6;11(5):e00852-20. doi: 10.1128/mBio.00852-20. mBio. 2020. PMID: 33024031 Free PMC article. - The parasitophorous vacuole membrane of Toxoplasma gondii is targeted for disruption by ubiquitin-like conjugation systems of autophagy.
Choi J, Park S, Biering SB, Selleck E, Liu CY, Zhang X, Fujita N, Saitoh T, Akira S, Yoshimori T, Sibley LD, Hwang S, Virgin HW. Choi J, et al. Immunity. 2014 Jun 19;40(6):924-35. doi: 10.1016/j.immuni.2014.05.006. Epub 2014 Jun 12. Immunity. 2014. PMID: 24931121 Free PMC article. - Autophagy in immunity against Toxoplasma gondii.
Subauste CS. Subauste CS. Curr Top Microbiol Immunol. 2009;335:251-65. doi: 10.1007/978-3-642-00302-8_12. Curr Top Microbiol Immunol. 2009. PMID: 19802569 Review. - Exposing Toxoplasma gondii hiding inside the vacuole: a role for GBPs, autophagy and host cell death.
Saeij JP, Frickel EM. Saeij JP, et al. Curr Opin Microbiol. 2017 Dec;40:72-80. doi: 10.1016/j.mib.2017.10.021. Epub 2017 Nov 12. Curr Opin Microbiol. 2017. PMID: 29141239 Free PMC article. Review.
Cited by
- MicroRNA expression profiling of Leishmania donovani-infected host cells uncovers the regulatory role of MIR30A-3p in host autophagy.
Singh AK, Pandey RK, Shaha C, Madhubala R. Singh AK, et al. Autophagy. 2016 Oct 2;12(10):1817-1831. doi: 10.1080/15548627.2016.1203500. Epub 2016 Jul 26. Autophagy. 2016. PMID: 27459332 Free PMC article. - The bacterial and cellular determinants controlling the recruitment of mTOR to the Salmonella-containing vacuole.
Tattoli I, Philpott DJ, Girardin SE. Tattoli I, et al. Biol Open. 2012 Dec 15;1(12):1215-25. doi: 10.1242/bio.20122840. Epub 2012 Oct 4. Biol Open. 2012. PMID: 23259056 Free PMC article. - Phytohormones regulate asexual Toxoplasma gondii replication.
Wagner T, Bangoura B, Wiedmer S, Daugschies A, Dunay IR. Wagner T, et al. Parasitol Res. 2023 Dec;122(12):2835-2846. doi: 10.1007/s00436-023-07968-3. Epub 2023 Sep 19. Parasitol Res. 2023. PMID: 37725257 - Are reactive oxygen species always detrimental to pathogens?
Paiva CN, Bozza MT. Paiva CN, et al. Antioxid Redox Signal. 2014 Feb 20;20(6):1000-37. doi: 10.1089/ars.2013.5447. Epub 2013 Oct 26. Antioxid Redox Signal. 2014. PMID: 23992156 Free PMC article. Review. - Toxoplasma-induced autophagy: a window into nutritional futile cycles in mammalian cells?
Orlofsky A. Orlofsky A. Autophagy. 2009 Apr;5(3):404-6. doi: 10.4161/auto.5.3.7807. Epub 2009 Apr 9. Autophagy. 2009. PMID: 19305153 Free PMC article.
References
- Mizushima, N., and Klionsky, D. J. (2007) Annu. Rev. Nutr. 27 19-40 - PubMed
- Wullschleger, S., Loewith, R., and Hall, M. N. (2006) Cell 124 471-484 - PubMed
- Py, B. F., Lipinski, M. M., and Yuan, J. Y. (2007) Autophagy 3 117-125 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI039454/AI/NIAID NIH HHS/United States
- R01 AI039454-13/AI/NIAID NIH HHS/United States
- R01 AI039454-12/AI/NIAID NIH HHS/United States
- AI-55358/AI/NIAID NIH HHS/United States
- AI-39454/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous