Protein O-GlcNAcylation: a new signaling paradigm for the cardiovascular system - PubMed (original) (raw)
Protein O-GlcNAcylation: a new signaling paradigm for the cardiovascular system
Boglarka Laczy et al. Am J Physiol Heart Circ Physiol. 2009 Jan.
Abstract
The posttranslational modification of serine and threonine residues of nuclear and cytoplasmic proteins by the O-linked attachment of the monosaccharide beta-N-acetylglucosamine (O-GlcNAc) is a highly dynamic and ubiquitous protein modification. Protein O-GlcNAcylation is rapidly emerging as a key regulator of critical biological processes including nuclear transport, translation and transcription, signal transduction, cytoskeletal reorganization, proteasomal degradation, and apoptosis. Increased levels of O-GlcNAc have been implicated as a pathogenic contributor to glucose toxicity and insulin resistance, which are both major hallmarks of diabetes mellitus and diabetes-related cardiovascular complications. Conversely, there is a growing body of data demonstrating that the acute activation of O-GlcNAc levels is an endogenous stress response designed to enhance cell survival. Reports on the effect of altered O-GlcNAc levels on the heart and cardiovascular system have been growing rapidly over the past few years and have implicated a role for O-GlcNAc in contributing to the adverse effects of diabetes on cardiovascular function as well as mediating the response to ischemic injury. Here, we summarize our present understanding of protein O-GlcNAcylation and its effect on the regulation of cardiovascular function. We examine the pathways regulating protein O-GlcNAcylation and discuss, in more detail, our understanding of the role of O-GlcNAc in both mediating the adverse effects of diabetes as well as its role in mediating cellular protective mechanisms in the cardiovascular system. In addition, we also explore the parallels between O-GlcNAc signaling and redox signaling, as an alternative paradigm for understanding the role of O-GlcNAcylation in regulating cell function.
Figures
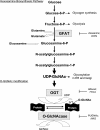
Fig. 1.
The hexosamine biosynthesis pathway (HBP) and protein _O_-GlcNAcylation. Glucose imported into cells is rapidly phosphorylated to glucose-6-phosphate (glucose-6-P) and converted to fructose-6-phosphate (fructose-6-P), which is metabolized to glucosamine-6-phosphate by
l
-glutamine-
d
-fructose 6-phosphate amidotransferase (GFAT), resulting in the synthesis of UDP-_N_-acetylglucosamine (UDP-GlcNAc). GFAT can be inhibited by the glutamine analogs 6-diazo-5-oxo-
l
-norleucine (DON) and _O_-diazoacetyl-
l
-serine (azaserine). Flux through the HBP can be increased with glucosamine, which bypasses GFAT. UDP-GlcNAc is a sugar donor for classical glycosylation reactions in the endoplasmic reticulum (ER) and Golgi apparatus and is also the obligatory substrate for uridine-diphospho-_N_-acetylglucosamine:polypeptide β-_N_-acetylglucosaminyltransferase (OGT), leading to the formation of _O_-linked β-_N_-acetylglucosamine (_O_-GlcNAc)-modified proteins. β-_N_-acetylglucosaminidase (_O_-GlcNAcase) catalyzes the removal of _O_-GlcNAc from proteins. The level of _O_-GlcNAc on proteins can be blocked by inhibiting OGT with the uridine analog alloxan or with 2[(4-chlorophenyl)imino]tetrahydro-4-oxo-3-[2-tricyclo(3.3.1.13.7)dec-1-ylethel] (TTO4), whereas _O_-GlcNAcylation of proteins can be rapidly increased by inhibiting _O_-GlcNAcase with _O_-(2-acetamido-2-deoxy-
d
-glucopyranosylidene)amino-_N_-phenylcarbamate (PUGNAc) or with 1,2 dideoxy-2-methyl-
d
-glucopyranoso(2,1-
d
)-2-thiazoline (NAG-thiazoline). S/T, serine/threonine.
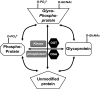
Fig. 2.
The interaction between _O_-GlcNAcylation and _O_-phosphorylation. Analogous to phosphorylation, _O_-GlcNAcylation is a dynamic posttranslational modification occurring on serine/threonine residues of proteins. For a subset of cellular proteins, there is a competitive relationship between _O_-GlcNAc and _O_-phosphate for the same serine/threonine residues, although there can be adjacent or multiple occupancy for phosphorylation and _O_-GlcNAcylation on the same protein. The combination of _O_-phosphate and _O_-GlcNAc modifications creates molecular diversity by altering specific protein sites that are involved in signaling events. Thus, this complex interplay between phosphorylation and _O_-GlcNAcylation can dynamically regulate protein functions and modulate critical signaling pathways. OGA, β-_N_-acetylglucosaminidase. [Modified from Zachara and Hart (226).]
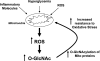
Fig. 3.
Relationship between mitochondrial oxidative stress and _O_-GlcNAcylation. Increased production of mitochondrial ROS induced either by inflammatory mediators, hyperglycemic condition (e.g., diabetic milieu), or oxidant stress leads to an increase in _O_-GlcNAc levels. Increased _O_-GlcNAcylation of mitochondrial proteins (e.g., the voltage-dependent anion channel) in turn protects cells against lethal damage by increasing mitochondrial stability and tolerance in response to oxidative stress stimuli.
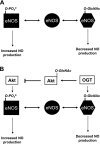
Fig. 4.
Cross-talk between _O_-GlcNAc, phosphorylation, and redox signaling. A: phosphorylation of endothelial nitric oxide (NO) synthase (eNOS) at Ser1177 results in increased eNOS activity and NO production, whereas _O_-GlcNAcylation of the same site leads to decreased enzyme activity and NO production. B: theoretically, _O_-GlcNAc-dependent reactions are not limited to interactions with specific proteins but rather act to regulate an entire pathway. For example, _O_-GlcNAc modification of eNOS decreases its activity and NO production. At the same site, Akt-mediated phosphorylation and activation of eNOS leads to increased NO-production. However, Akt is also subject to _O_-GlcNAcylation, which reduces its activity, thereby inhibiting eNOS phosphorylation and NO production.
Similar articles
- The hexosamine signaling pathway: deciphering the "O-GlcNAc code".
Love DC, Hanover JA. Love DC, et al. Sci STKE. 2005 Nov 29;2005(312):re13. doi: 10.1126/stke.3122005re13. Sci STKE. 2005. PMID: 16317114 Review. - Protein O-linked β-N-acetylglucosamine: a novel effector of cardiomyocyte metabolism and function.
Darley-Usmar VM, Ball LE, Chatham JC. Darley-Usmar VM, et al. J Mol Cell Cardiol. 2012 Mar;52(3):538-49. doi: 10.1016/j.yjmcc.2011.08.009. Epub 2011 Aug 22. J Mol Cell Cardiol. 2012. PMID: 21878340 Free PMC article. Review. - Role of protein O-linked N-acetyl-glucosamine in mediating cell function and survival in the cardiovascular system.
Fülöp N, Marchase RB, Chatham JC. Fülöp N, et al. Cardiovasc Res. 2007 Jan 15;73(2):288-97. doi: 10.1016/j.cardiores.2006.07.018. Epub 2006 Jul 29. Cardiovasc Res. 2007. PMID: 16970929 Free PMC article. Review. - The role of protein O-linked beta-N-acetylglucosamine in mediating cardiac stress responses.
Chatham JC, Marchase RB. Chatham JC, et al. Biochim Biophys Acta. 2010 Feb;1800(2):57-66. doi: 10.1016/j.bbagen.2009.07.004. Epub 2009 Jul 14. Biochim Biophys Acta. 2010. PMID: 19607882 Free PMC article. Review. - Post-translational protein modification by O-linked N-acetyl-glucosamine: its role in mediating the adverse effects of diabetes on the heart.
McLarty JL, Marsh SA, Chatham JC. McLarty JL, et al. Life Sci. 2013 Mar 28;92(11):621-7. doi: 10.1016/j.lfs.2012.08.006. Epub 2012 Aug 11. Life Sci. 2013. PMID: 22985933 Free PMC article. Review.
Cited by
- Effects of rosuvastatin combined with fasudil therapy on rabbits with dyslipidemia.
Li Z, Lian H, Liang Q, Zeng F, Zheng D. Li Z, et al. Lipids Health Dis. 2015 May 28;14:52. doi: 10.1186/s12944-015-0050-1. Lipids Health Dis. 2015. PMID: 26018523 Free PMC article. - O-GlcNAc signaling in the cardiovascular system.
Ngoh GA, Facundo HT, Zafir A, Jones SP. Ngoh GA, et al. Circ Res. 2010 Jul 23;107(2):171-85. doi: 10.1161/CIRCRESAHA.110.224675. Circ Res. 2010. PMID: 20651294 Free PMC article. Review. - Overview: the maturing of proteomics in cardiovascular research.
Van Eyk JE. Van Eyk JE. Circ Res. 2011 Feb 18;108(4):490-8. doi: 10.1161/CIRCRESAHA.110.226894. Circ Res. 2011. PMID: 21335431 Free PMC article. Review. - Detection and analysis of proteins modified by O-linked N-acetylglucosamine.
Zachara NE, Vosseller K, Hart GW. Zachara NE, et al. Curr Protoc Mol Biol. 2011 Jul;Chapter 17:Unit 17.6. doi: 10.1002/0471142727.mb1706s95. Curr Protoc Mol Biol. 2011. PMID: 21732316 Free PMC article. - Cytoplasmic Citrate Flux Modulates the Immune Stimulatory NKG2D Ligand MICA in Cancer Cells.
Møller SH, Mellergaard M, Madsen M, Bermejo AV, Jepsen SD, Hansen MH, Høgh RI, Aldana BI, Desler C, Rasmussen LJ, Sustarsic EG, Gerhart-Hines Z, Daskalaki E, Wheelock CE, Hiron TK, Lin D, O'Callaghan CA, Wandall HH, Andresen L, Skov S. Møller SH, et al. Front Immunol. 2020 Aug 11;11:1968. doi: 10.3389/fimmu.2020.01968. eCollection 2020. Front Immunol. 2020. PMID: 32849657 Free PMC article.
References
- Abe T, Ohga Y, Tabayashi N, Kobayashi S, Sakata S, Misawa H, Tsuji T, Kohzuki H, Suga H, Taniguchi S, Takaki M. Left ventricular diastolic dysfunction in type 2 diabetes mellitus model rats. Am J Physiol Heart Circ Physiol 282: H138–H148, 2002. - PubMed
- Ahmed N, Thornalley PJ. Advanced glycation endproducts: what is their relevance to diabetic complications? Diabetes Obes Metab 9: 233–245, 2007. - PubMed
- An D, Rodrigues B. Role of changes in cardiac metabolism in development of diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol 291: H1489–H1506, 2006. - PubMed
- Armoundas AA, Rose J, Aggarwal R, Stuyvers BD, O'Rourke B, Kass DA, Marban E, Shorofsky SR, Tomaselli GF, Balke WC. Cellular and molecular determinants of altered Ca2+ handling in the failing rabbit heart: primary defects in SR Ca2+ uptake and release mechanisms. Am J Physiol Heart Circ Physiol 292: H1607–H1618, 2007. - PMC - PubMed
- Asbun J, Manso AM, Villarreal FJ. Profibrotic influence of high glucose concentration on cardiac fibroblast functions: effects of losartan and vitamin E. Am J Physiol Heart Circ Physiol 288: H227–H234, 2005. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL-067464/HL/NHLBI NIH HHS/United States
- HL-077100/HL/NHLBI NIH HHS/United States
- R01 HL087980/HL/NHLBI NIH HHS/United States
- T32 HL007457/HL/NHLBI NIH HHS/United States
- R01 DK075865/DK/NIDDK NIH HHS/United States
- T-32-HL-07457/HL/NHLBI NIH HHS/United States
- R01 HL079364/HL/NHLBI NIH HHS/United States
- R01 HL075211/HL/NHLBI NIH HHS/United States
- P50 HL077100/HL/NHLBI NIH HHS/United States
- HL-079364/HL/NHLBI NIH HHS/United States
- HL-075211/HL/NHLBI NIH HHS/United States
- R01 HL067464/HL/NHLBI NIH HHS/United States
- HL-087980/HL/NHLBI NIH HHS/United States
- DK-075865/DK/NIDDK NIH HHS/United States
- R01 DK075865-03/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous