Live-cell imaging of dendritic spines by STED microscopy - PubMed (original) (raw)
Live-cell imaging of dendritic spines by STED microscopy
U Valentin Nägerl et al. Proc Natl Acad Sci U S A. 2008.
Abstract
Time lapse fluorescence imaging has become one of the most important approaches in neurobiological research. In particular, both confocal and two-photon microscopy have been used to study activity-dependent changes in synaptic morphology. However, the diffraction-limited resolution of light microscopy is often inadequate, forcing researchers to complement the live cell imaging strategy by EM. Here, we report on the first use of a far-field optical technique with subdiffraction resolution to noninvasively image activity-dependent morphological plasticity of dendritic spines. Specifically we show that time lapse stimulated emission depletion imaging of dendritic spines of YFP-positive hippocampal neurons in organotypic slices outperforms confocal microscopy in revealing important structural details. The technique substantially improves the quantification of morphological parameters, such as the neck width and the curvature of the heads of spines, which are thought to play critical roles for the function and plasticity of synaptic connections.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
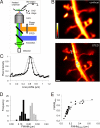
Fig. 1.
STED microscopy of dendritic structures. (A) Schematic representation of the experimental setup. (B) Dendritic spines imaged either by confocal (Top) or STED (Bottom) microscopy. (Scale bar, 0.5 μm.) Note that pixel intensity representation is not saturated over the regions of interest, i.e., at the spine necks. (C) Normalized line profile of pixel intensity across the neck of a spine as imaged in confocal (gray curve) or STED mode (black curve); line width: four pixels, indicated by shaded bar in B. (D) Frequency histogram of FWHM values for spine necks imaged in confocal (gray bars) and STED (black bars) modes. (E) Plot of ratio of FWHM values in STED over confocal modes as a function of FWHM values of spine necks and dendritic shafts imaged in the STED mode.
Fig. 2.
High-resolution 3D STED imaging of dendritic structures. (A) Volume reconstruction of image stack data; the panels show individual image sections acquired at the z level indicated (Δz = 0.5 μm). (B) Another example of a reconstructed stretch of dendrite (Δz = 0.25 μm), the lateral pixel size was 29 nm/pixel in both cases. (Scale bars, 1 μm.)
Fig. 3.
Time lapse STED imaging of dendritic structures. (A and B) Series of image frames of YFP-labeled dendritic spines acquired by STED microscopy at 40 sec/frame (20 frames were acquired in total) under unstimulated conditions. Arrows indicate cup-like shapes of spine heads; STED pulse peak intensity 400 MW/cm2 (A) and 215 MW/cm2 (B). (Scale bars, 1 μm.) (C) Time course of fluorescence intensity in the heads of spines as a function of consecutively acquired image frames (20 sec/frame).
Fig. 4.
STED imaging of activity-dependent postsynaptic morphological plasticity. (A–D) Examples of structural changes of dendritic spines after plasticity-inducing stimulation using the chemical LTP protocol. The first frame was taken before the stimulation, while all subsequent ones at the times indicated (h:min). Arrows indicate sites of structural rearrangements. (Scale bars, 0.5 μm.)
Similar articles
- Stimulated emission depletion (STED) imaging of dendritic spines in living hippocampal slices.
Willig KI, Nägerl UV. Willig KI, et al. Cold Spring Harb Protoc. 2012 May 1;2012(5):pdb.prot069260. doi: 10.1101/pdb.prot069260. Cold Spring Harb Protoc. 2012. PMID: 22550296 - Considerations for Imaging and Analyzing Neural Structures by STED Microscopy.
Lenz MO, Tønnesen J. Lenz MO, et al. Methods Mol Biol. 2019;1941:29-46. doi: 10.1007/978-1-4939-9077-1_3. Methods Mol Biol. 2019. PMID: 30707425 - Stimulated emission depletion (STED) microscopy reveals nanoscale defects in the developmental trajectory of dendritic spine morphogenesis in a mouse model of fragile X syndrome.
Wijetunge LS, Angibaud J, Frick A, Kind PC, Nägerl UV. Wijetunge LS, et al. J Neurosci. 2014 Apr 30;34(18):6405-12. doi: 10.1523/JNEUROSCI.5302-13.2014. J Neurosci. 2014. PMID: 24790210 Free PMC article. - Super-resolution STED microscopy in live brain tissue.
Calovi S, Soria FN, Tønnesen J. Calovi S, et al. Neurobiol Dis. 2021 Aug;156:105420. doi: 10.1016/j.nbd.2021.105420. Epub 2021 Jun 5. Neurobiol Dis. 2021. PMID: 34102277 Review. - Dendritic spines and long-term plasticity.
Segal M. Segal M. Nat Rev Neurosci. 2005 Apr;6(4):277-84. doi: 10.1038/nrn1649. Nat Rev Neurosci. 2005. PMID: 15803159 Review.
Cited by
- In vivo super-resolution of the brain - How to visualize the hidden nanoplasticity?
Willig KI. Willig KI. iScience. 2022 Aug 17;25(9):104961. doi: 10.1016/j.isci.2022.104961. eCollection 2022 Sep 16. iScience. 2022. PMID: 36093060 Free PMC article. Review. - Multiscale fluorescence imaging of living samples.
Wu Y, Shroff H. Wu Y, et al. Histochem Cell Biol. 2022 Oct;158(4):301-323. doi: 10.1007/s00418-022-02147-4. Epub 2022 Aug 29. Histochem Cell Biol. 2022. PMID: 36036808 Free PMC article. Review. - The next generation of approaches to investigate the link between synaptic plasticity and learning.
Humeau Y, Choquet D. Humeau Y, et al. Nat Neurosci. 2019 Oct;22(10):1536-1543. doi: 10.1038/s41593-019-0480-6. Epub 2019 Sep 2. Nat Neurosci. 2019. PMID: 31477899 Review. - Projection neuron circuits resolved using correlative array tomography.
Oberti D, Kirschmann MA, Hahnloser RH. Oberti D, et al. Front Neurosci. 2011 Apr 12;5:50. doi: 10.3389/fnins.2011.00050. eCollection 2011. Front Neurosci. 2011. PMID: 21519397 Free PMC article. - From whole organism to ultrastructure: progress in axonal imaging for decoding circuit development.
Weaver CJ, Poulain FE. Weaver CJ, et al. Development. 2021 Sep 15;148(18):dev199717. doi: 10.1242/dev.199717. Epub 2021 Jul 30. Development. 2021. PMID: 34328171 Free PMC article. Review.
References
- Denk W, Strickler JH, Webb WW. 2-Photon Laser Scanning Fluorescence Microscopy. Science. 1990;248:73–76. - PubMed
- Yuste R, Bonhoeffer T. Morphological changes in dendritic spines associated with long-term synaptic plasticity. Annu Rev Neurosci. 2001;24:1071–1089. - PubMed
- Engert F, Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399:66–70. - PubMed
- Maletic-Savatic M, Malinow R, Svoboda K. Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science. 1999;283:1923–1927. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources