Riluzole treatment, survival and diagnostic criteria in Parkinson plus disorders: the NNIPPS study - PubMed (original) (raw)
Clinical Trial
. 2009 Jan;132(Pt 1):156-71.
doi: 10.1093/brain/awn291. Epub 2008 Nov 23.
Collaborators, Affiliations
- PMID: 19029129
- PMCID: PMC2638696
- DOI: 10.1093/brain/awn291
Clinical Trial
Riluzole treatment, survival and diagnostic criteria in Parkinson plus disorders: the NNIPPS study
Gilbert Bensimon et al. Brain. 2009 Jan.
Abstract
Parkinson plus diseases, comprising mainly progressive supranuclear palsy (PSP) and multiple system atrophy (MSA) are rare neurodegenerative conditions. We designed a double-blind randomized placebo-controlled trial of riluzole as a potential disease-modifying agent in Parkinson plus disorders (NNIPPS: Neuroprotection and Natural History in Parkinson Plus Syndromes). We analysed the accuracy of our clinical diagnostic criteria, and studied prognostic factors for survival. Patients with an akinetic-rigid syndrome diagnosed as having PSP or MSA according to modified consensus diagnostic criteria were considered for inclusion. The psychometric validity (convergent and predictive) of the NNIPPS diagnostic criteria were tested prospectively by clinical and pathological assessments. The study was powered to detect a 40% decrease in relative risk of death within PSP or MSA strata. Patients were randomized to riluzole or matched placebo daily and followed up to 36 months. The primary endpoint was survival. Secondary efficacy outcomes were rates of disease progression assessed by functional measures. A total of 767 patients were randomized and 760 qualified for the Intent to Treat (ITT) analysis, stratified at entry as PSP (362 patients) or MSA (398 patients). Median follow-up was 1095 days (range 249-1095). During the study, 342 patients died and 112 brains were examined for pathology. NNIPPS diagnostic criteria showed for both PSP and MSA excellent convergent validity with the investigators' assessment of diagnostic probability (point-biserial correlation: MSA r(pb) = 0.93, P < 0.0001; PSP, r(pb) = 0.95, P < 0.0001), and excellent predictive validity against histopathology [sensitivity and specificity (95% CI) for PSP 0.95 (0.88-0.98) and 0.84 (0.77-0.87); and for MSA 0.96 (0.88-0.99) and 0.91 (0.86-0.93)]. There was no evidence of a drug effect on survival in the PSP or MSA strata (3 year Kaplan-Meier estimates PSP-riluzole: 0.51, PSP-placebo: 0.50; MSA-riluzole: 0.53, MSA-placebo: 0.58; P = 0.66 and P = 0.48 by the log-rank test, respectively), or in the population as a whole (P = 0.42, by the stratified-log-rank test). Likewise, rate of progression was similar in both treatment groups. There were no unexpected adverse effects of riluzole, and no significant safety concerns. Riluzole did not have a significant effect on survival or rate of functional deterioration in PSP or MSA, although the study reached over 80% power to detect the hypothesized drug effect within strata. The NNIPPS diagnostic criteria were consistent and valid. They can be used to distinguish between PSP and MSA with high accuracy, and should facilitate research into these conditions relatively early in their evolution.
Trial registration: ClinicalTrials.gov NCT00211224.
Figures
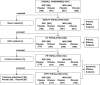
Fig. 1
Trial Flow Chart. At the selection stage, patients were assigned to either the MSA or PSP strata according to the NNIPPS diagnostic criteria. Following Inclusion, patients within each stratum were randomly allocated to either the riluzole or placebo group on 1:1 ratio and followed-up 3 monthly for 36 months in double-blind fashion. Arrows indicate the time of each assessment.
Fig. 2
NNIPPS populations in analyses.
Fig. 3
Kaplan–Meier survival curves of riluzole and placebo groups in PSP and MSA strata.
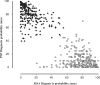
Fig. 4
Convergent validity of NNIPPS Diagnostic Criteria with Investigators’ Diagnostic Probability (VAS). At the inclusion visit, following patients’ assignment to strata using the NNIPPS diagnostic criteria, investigators were asked to evaluate the probability of each diagnosis (PSP, MSA), using a 100 mm VAS. All 760 patients are plotted on the graph according to the probability score on each VAS (PSP-vertical axis, MSA-horizontal axis). Solid diamonds represent patients included in the PSP stratum; White circles represent patients included in the MSA stratum. Convergent validity of the NNIPPS inclusion criteria with the investigators’ assessment of diagnostic probability was tested with the point-biserial correlation. MSA, _r_pb = 0.93 (P < 0.0001), PSP, _r_pb = 0.95, (P < 0.0001).
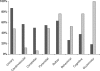
Fig. 5
Syndrome profile of patients in PSP and MSA Strata. At the inclusion visit, and based on the clinical neurological assessments, investigators were asked to describe the syndrome profile of the patients using a systematic (yes/no) questionnaire. Each bar represents the percentage of patients within each stratum positive for a given syndrome. Black bars represent the MSA stratum, grey bars the PSP stratum. The akinetic rigid syndrome was a mandatory inclusion criterion for both strata and therefore is not represented.
Similar articles
- Disease severity and progression in progressive supranuclear palsy and multiple system atrophy: validation of the NNIPPS--Parkinson Plus Scale.
Payan CA, Viallet F, Landwehrmeyer BG, Bonnet AM, Borg M, Durif F, Lacomblez L, Bloch F, Verny M, Fermanian J, Agid Y, Ludolph AC, Leigh PN, Bensimon G; NNIPPS Study Group. Payan CA, et al. PLoS One. 2011;6(8):e22293. doi: 10.1371/journal.pone.0022293. Epub 2011 Aug 4. PLoS One. 2011. PMID: 21829612 Free PMC article. - Predictors of survival in progressive supranuclear palsy and multiple system atrophy: a systematic review and meta-analysis.
Glasmacher SA, Leigh PN, Saha RA. Glasmacher SA, et al. J Neurol Neurosurg Psychiatry. 2017 May;88(5):402-411. doi: 10.1136/jnnp-2016-314956. Epub 2017 Mar 1. J Neurol Neurosurg Psychiatry. 2017. PMID: 28250027 Review. - Montreal Cognitive Assessment (MoCA) and Mini-Mental State Examination (MMSE) performance in progressive supranuclear palsy and multiple system atrophy.
Fiorenzato E, Weis L, Falup-Pecurariu C, Diaconu S, Siri C, Reali E, Pezzoli G, Bisiacchi P, Antonini A, Biundo R. Fiorenzato E, et al. J Neural Transm (Vienna). 2016 Dec;123(12):1435-1442. doi: 10.1007/s00702-016-1589-3. Epub 2016 Jun 22. J Neural Transm (Vienna). 2016. PMID: 27334897 - Accuracy of the National Institute for Neurological Disorders and Stroke/Society for Progressive Supranuclear Palsy and neuroprotection and natural history in Parkinson plus syndromes criteria for the diagnosis of progressive supranuclear palsy.
Respondek G, Roeber S, Kretzschmar H, Troakes C, Al-Sarraj S, Gelpi E, Gaig C, Chiu WZ, van Swieten JC, Oertel WH, Höglinger GU. Respondek G, et al. Mov Disord. 2013 Apr;28(4):504-9. doi: 10.1002/mds.25327. Epub 2013 Feb 21. Mov Disord. 2013. PMID: 23436751 - Therapeutic advances in multiple system atrophy and progressive supranuclear palsy.
Poewe W, Mahlknecht P, Krismer F. Poewe W, et al. Mov Disord. 2015 Sep 15;30(11):1528-38. doi: 10.1002/mds.26334. Epub 2015 Jul 30. Mov Disord. 2015. PMID: 26227071 Review.
Cited by
- Efficacy and safety of riluzole for treating motor function in rare dyskinesia syndromes: a systematic review with meta-analysis.
Wang R, Fang H, Shen Y, Qiu M. Wang R, et al. J Int Med Res. 2024 Sep;52(9):3000605241276489. doi: 10.1177/03000605241276489. J Int Med Res. 2024. PMID: 39340255 Free PMC article. - Ethnic Differences in Atypical Parkinsonism-is South Asian PSP Different?
Balint B, Neo S, Magrinelli F, Mulroy E, Latorre A, Stamelou M, Morris HR, Batla A, Bhatia KP. Balint B, et al. Mov Disord Clin Pract. 2024 Nov;11(11):1355-1364. doi: 10.1002/mdc3.14182. Epub 2024 Aug 7. Mov Disord Clin Pract. 2024. PMID: 39113437 Free PMC article. - Pharmacotherapies for the Treatment of Progressive Supranuclear Palsy: A Narrative Review.
Dunning EE, Decourt B, Zawia NH, Shill HA, Sabbagh MN. Dunning EE, et al. Neurol Ther. 2024 Aug;13(4):975-1013. doi: 10.1007/s40120-024-00614-9. Epub 2024 May 14. Neurol Ther. 2024. PMID: 38743312 Free PMC article. Review. - Multiple system atrophy: an update and emerging directions of biomarkers and clinical trials.
Liu M, Wang Z, Shang H. Liu M, et al. J Neurol. 2024 May;271(5):2324-2344. doi: 10.1007/s00415-024-12269-5. Epub 2024 Mar 14. J Neurol. 2024. PMID: 38483626 Free PMC article. Review. - Recent Advances in Clinical Trials in Multiple System Atrophy.
Bendetowicz D, Fabbri M, Sirna F, Fernagut PO, Foubert-Samier A, Saulnier T, Le Traon AP, Proust-Lima C, Rascol O, Meissner WG. Bendetowicz D, et al. Curr Neurol Neurosci Rep. 2024 Apr;24(4):95-112. doi: 10.1007/s11910-024-01335-0. Epub 2024 Feb 28. Curr Neurol Neurosci Rep. 2024. PMID: 38416311 Review.
References
- Albers DS, Augood SJ. New insights into progressive supranuclear palsy. Trends Neurosci. 2001;24:347–53. - PubMed
- Albin RL, Greenamyre JT. Alternative excitotoxic hypotheses. Neurology. 1992;42:733–8. - PubMed
- Allison P. Survival Analysis using the SAS System – A Practical Guide. SAS Institute Inc.; 1995.
- American Psychological Association. Standard for educational and psychological testing. Washington DC: American Psychological Association ed.; 1985.
- Attia J. Moving beyond sensitivity and specificity: Using likelihood ratios to help interpret diagnostic tests. Australian Prescriber. 2003;26:111–13.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous