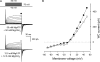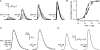Voltage dependence of ATP secretion in mammalian taste cells - PubMed (original) (raw)
Voltage dependence of ATP secretion in mammalian taste cells
Roman A Romanov et al. J Gen Physiol. 2008 Dec.
Abstract
Mammalian type II taste cells release the afferent neurotransmitter adenosine triphosphate (ATP) through ATP-permeable ion channels, most likely to be connexin (Cx) and/or pannexin hemichannels. Here, we show that ion channels responsible for voltage-gated (VG) outward currents in type II cells are ATP permeable and demonstrate a strong correlation between the magnitude of the VG current and the intensity of ATP release. These findings suggest that slowly deactivating ion channels transporting the VG outward currents can also mediate ATP secretion in type II cells. In line with this inference, we studied a dependence of ATP secretion on membrane voltage with a cellular ATP sensor using different pulse protocols. These were designed on the basis of predictions of a model of voltage-dependent transient ATP efflux. Consistently with curves that were simulated for ATP release mediated by ATP-permeable channels deactivating slowly, the bell-like and Langmuir isotherm-like potential dependencies were characteristic of ATP secretion obtained for prolonged and short electrical stimulations of taste cells, respectively. These observations strongly support the idea that ATP is primarily released via slowly deactivating channels. Depolarizing voltage pulses produced negligible Ca(2+) transients in the cytoplasm of cells releasing ATP, suggesting that ATP secretion is mainly governed by membrane voltage under our recording conditions. With the proviso that natural connexons and pannexons are kinetically similar to exogenously expressed hemichannels, our findings suggest that VG ATP release in type II cells is primarily mediated by Cx hemichannels.
Figures
Figure 1.
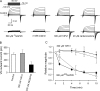
Effects of hemichannel blockers on VG outward currents and ATP secretion in taste cells of the type II. (A) Representative recordings of VG currents in control (top panels) and after a 10-min incubation with the Cx mimetic peptide 43GAP26 (500 μM; n = 21), 3 mM octanol (n = 11), Px mimetic peptide 10Px1 (300 μM; n = 9), and 20 μM carbenoxolone (n = 7) in the bath. The traces presented in different panels were obtained from four different cells, which were held at −70 mV and polarized by 100-ms voltage pulses between −100 and 50 mV with the 10-mV decrement, as shown in the top left panel. (B) VG currents on the 100-ms depolarization to 20 mV recorded in control cells (n = 32) and in cells preincubated either with 10Px1 (300 μM; n = 7) or with 43GAP26 (300 μM; n = 12) for 30 min. The difference between the averaged current magnitudes in control and in the presence of 10Px1 is not statistically significant at the P < 0.05 confidence level. (C) Evolution of steady-state VG currents measured in the end of voltage pulses (•, ▾) and ATP sensor responses (○, ▵) after the addition of 43GAP26 (300 μM; •, ○; n = 14), and Px1 mimetic peptide 10Px1 (300 μM; ▾, ▵; n = 9) to the bath at t = 0. The VG currents were elicited by the 100-ms depolarization of taste cells from −70 to 0 mV that did not stimulate detectable ATP efflux. The secretion of ATP was elicited by the 2-s depolarization of taste cells to 10 mV. In B and C, the data are presented as a mean ± SD. In all cases, VG currents were recorded with 140 mM CsCl in the recording pipette and 140 mM NaCl in the bath using the perforated patch approach.
Figure 2.
VG currents in taste cells dialyzed and perfused with MgATP+Mg(OH)2. (A) Representative WC currents (n = 4) recorded from the same cell perfused with 50 mM MgATP+Mg(OH)2 (middle) and with 12.5 mM MgATP+Mg(OH)2 (bottom). The currents were recorded with 50 mM MgATP+Mg(OH)2 in the recording pipette. Taste cells were patched in the bath solution containing 140 mM NaCl that was substituted for 50 mM MgATP+Mg(OH)2 after obtaining the WC mode. (B) Voltage current curves generated with 50 mM (•) and 12.5 mM (○) MgATP+Mg(OH)2 in the bath. With 50 mM MgATP+Mg(OH)2 both in the recording pipette and in the bath, WC currents did not reverse at 0 mV, as membrane voltage was not corrected for the liquid junction potential of ∼10 mV between solutions containing 140 mM NaCl and 50 mM MgATP+Mg(OH)2, respectively. Nearly the same junctional potential arose across the boundary between the solutions containing 140 mM NaCl and 12.5 mM MgATP+Mg(OH)2. Because the lifetime of stable WC preparations was very short with high MgATP+Mg(OH)2 in the pipette, neither serial resistance nor cell capacitance was compensated for in the presented recordings.
Figure 3.
ATP release on electrical stimulation is not accompanied by a change in intracellular Ca2+. (A and B) Representative simultaneous recordings of ionic currents and fluorescence in a type III cell loaded with 4 μM Fluo-4 (n = 6). The depolarization of the taste cell to −10 mV for 100 ms (A and B, top panels) elicited the VG Ca2+ current (A, bottom panel, dotted line) that produced the Ca2+ transient monitored as an increase in Fluo-4 fluorescence (B, bottom panel). A relative change in fluorescence intensity is expressed as ΔF/F0, where F0 is a value of Fluo-4 emission measured just before cell stimulation. (C and D) Representative concurrent recordings of ionic currents (middle panel) and Fluo-4 fluorescence (bottom panel) in a type II cell (n = 21). Unlike ionomycin, 2-s depolarization (top panel) did not affect intracellular Ca2+ in 17 of 21 type II cells (C). In 4 out of 21 cells, depolarization induced a small increase in Fluo-4 fluorescence (D). (E) Representative concurrent recordings of a membrane current and intracellular Ca2+ in a type II cell and an ATP sensor response (n = 9). The 5-s depolarization (top panel) of the taste cell produced no change in cytosolic Ca2+ (the second trace from the bottom) but triggered ATP release, thereby stimulating the ATP sensor (bottom panel). The recording conditions were as in Fig. 1.
Figure 4.
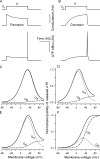
Simulations of hemichannel gating and voltage-dependent ATP efflux. (A and B) Transient integral conductance (middle panels) mediated by Px1 hemichannels (A) and Cx hemichannels (B) stimulated by the depolarizing voltage pulse from _V_h to V (top panel) for T seconds. The Px1 conductance was calculated in an arbitrary unit (AU) with Eqs. 7 and 9 at V = 10 mV, _V_h = −70 mV, _V_g = 50 mV, _V_g0 =13 mV, and _τ_a = _τ_d = _τ_i/50 = T/250. The Cx conductance was calculated with Eqs. 8 and 9 at V = 10 mV, _V_h = −70 mV, _V_g = 50 mV, _V_g0 = 13 mV, and _τ_d = _τ_a/10 =T/50. The related transient ATP efflux (bottom panels) was calculated by using Eqs. 7–9 and Eq. 6 with L = 1, z = 2, i.e., at _V_0 = 13 mV, r = 0. (C and D) Normalized quantity of ATP released via Px1 hemichannels versus membrane voltage calculated using Eqs. 11–13 at _τ_a = _τ_d = _τ_i/50 and with T = 50_τ_i (C) and T = 5_τ_i (D). (E and F) Normalized quantity of ATP released via Cx hemichannels versus membrane voltage calculated using Eqs. 11, 12, and 14 at _τ_d = _τ_a/10 and with T = 50_τ_a (E) and T = 5_τ_a (F). The details are in the text.
Figure 5.
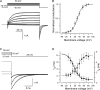
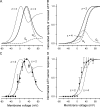
Voltage-dependent kinetics of VG currents mediated by hemichannels in a type II taste cell. (A) VG currents recorded at different voltages were fitted (•) with Eq. 15. (B) Normalized steady-state conductance _G_s/_G_max versus membrane voltage. For given voltage, _G_s (▵) was obtained by fitting a corresponding current trace with Eq. 15. To compare different experiments, _G_s was normalized to the maximal conductance _G_max obtained for a given cell. The solid line corresponds to Eq. 16 with _V_g = 31 mV and = 11 mV. (C) Tail currents (continuous lines) were approximated with Eq. 17 (•). (D) Potential dependence of the characteristic time of activation (▾) and deactivation (▪) of VG currents. The thick and thin lines correspond to Eqs. 18 and 19, respectively. In all cases, the recording conditions were as in Fig. 1. In B and D, the data are presented as a mean ± SD (n = 7).
Figure 6.
Dependence of ATP secretion on membrane voltage. (A and B) Calculated quantity of ATP released on 2-s (A) and 100-ms (B) electrical stimulations. The thick lines depict the total ATP quantity versus membrane voltage calculated as _Q_ATP = _Q_a + _Q_d using Eqs. 11, 12, and 14 with the following parameters: G0 = 1, r = 0, V_0 = R_T/zF = 13 mV at z = 2, _V_g and _V_g0 as in Eq. 16, and with _τ_a and _τ_d determined by Eqs. 18 and 19, respectively. The dotted and dashed curves correspond to Eqs. 11 and 12, respectively, at the parameters mentioned above. The thin curve describes _Q_ATP = _Q_a + _Q_d calculated with z = 4 and the other parameters as above. Each dependence in A and B was normalized to the maximal value of _Q_ATP. (C) Normalized response of the ATP sensor (•) versus voltage clamped on the plasma membrane of an assayed taste cell. The ATP responses were recorded at 2 s of depolarization of taste cells. The thick and thin curves were obtained by converting _Q_ATP calculated at z = 2 and z = 4 (thick and thin curves in A, respectively) into ATP sensor responses using Eq. 20 with = 0.49. Both experimental and simulated responses were normalized to the corresponding maximal value. (D) ATP responses (▾) recorded at the serial stimulation of taste cells by 100-ms pulses for 2 s. The thick and thin curves were obtained by converting _Q_ATP calculated at z = 2 and z = 4 (thick and thin curves in B) into ATP sensor responses using Eq. 20 with
= 0.49. The responses were normalized to a value of the maximal response. The experimental data are presented as a mean ± SD (n = 5–8). The recording conditions were as in Fig. 1.
Figure A1.
ATP-induced Ca2+ transients in Fluo-4–loaded COS-1 cells. (A) Superimposition of Ca2+ transients elicited by a 2-s application of 75–1,000 nM ATP in eight COS-1 cells assayed simultaneously. (B) Dose-response curves characterizing the whole population of tested COS-1 cells (•) and COS-1 cells selected as most sensitive to ATP (▾). The thick and thin lines represent the approximations of experimental dependencies with Eq. A1 at [_ATP_]1/2 = 160 nM and nH = 2 and [_ATP_]1/2 = 270 nM and nH = 1.5, respectively. (C; left) The superimposition of averaged (n = 4) responses to ATP applied at the concentration of 150 (dotted line), 300 (thick line), and 1,000 nM (thin line) for 2 s. Each response was normalized to its magnitude. (Right) The fit (○) of the averaged response to 300 nM ATP (solid line) with Eq. A2 at α = 0.32 s−1, β = 0.15 s−1, and _t_0 = 8.1 s. AU, arbitrary unit. (D) Averaged (n = 4) responses to 300 nM ATP applied for 2 (left panel) and 8 s (right panel). In C and D, all responses were recorded from COS-1 cells selected by their high sensitivity to ATP (thick lines in A). In all cases, cells were incubated in the basic extracellular solution and stimulated by bath application of ATP dissolved in the same solution.
Similar articles
- Action potentials and ion conductances in wild-type and CALHM1-knockout type II taste cells.
Ma Z, Saung WT, Foskett JK. Ma Z, et al. J Neurophysiol. 2017 May 1;117(5):1865-1876. doi: 10.1152/jn.00835.2016. Epub 2017 Feb 15. J Neurophysiol. 2017. PMID: 28202574 Free PMC article. - Intracellular Ca(2+) and TRPM5-mediated membrane depolarization produce ATP secretion from taste receptor cells.
Huang YA, Roper SD. Huang YA, et al. J Physiol. 2010 Jul 1;588(Pt 13):2343-50. doi: 10.1113/jphysiol.2010.191106. Epub 2010 May 24. J Physiol. 2010. PMID: 20498227 Free PMC article. - Amiloride-sensitive channels in type I fungiform taste cells in mouse.
Vandenbeuch A, Clapp TR, Kinnamon SC. Vandenbeuch A, et al. BMC Neurosci. 2008 Jan 2;9:1. doi: 10.1186/1471-2202-9-1. BMC Neurosci. 2008. PMID: 18171468 Free PMC article. - How do taste cells lacking synapses mediate neurotransmission? CALHM1, a voltage-gated ATP channel.
Taruno A, Matsumoto I, Ma Z, Marambaud P, Foskett JK. Taruno A, et al. Bioessays. 2013 Dec;35(12):1111-8. doi: 10.1002/bies.201300077. Epub 2013 Sep 17. Bioessays. 2013. PMID: 24105910 Free PMC article. Review. - Electrophysiology of islet cells.
Drews G, Krippeit-Drews P, Düfer M. Drews G, et al. Adv Exp Med Biol. 2010;654:115-63. doi: 10.1007/978-90-481-3271-3_7. Adv Exp Med Biol. 2010. PMID: 20217497 Review.
Cited by
- The role of GABA in modulation of taste signaling within the taste bud.
Mikami A, Huang H, Hyodo A, Horie K, Yasumatsu K, Ninomiya Y, Mitoh Y, Iida S, Yoshida R. Mikami A, et al. Pflugers Arch. 2024 Nov;476(11):1761-1775. doi: 10.1007/s00424-024-03007-x. Epub 2024 Aug 29. Pflugers Arch. 2024. PMID: 39210062 Free PMC article. - Pattern Distribution of Connexins in the Ortho- and Parakeratinized Epithelium of the Lingual Mucosa in Birds.
Skieresz-Szewczyk K, Jackowiak H. Skieresz-Szewczyk K, et al. Cells. 2023 Jul 4;12(13):1776. doi: 10.3390/cells12131776. Cells. 2023. PMID: 37443811 Free PMC article. - Purinergic neurotransmission in the gustatory system.
Finger T, Kinnamon S. Finger T, et al. Auton Neurosci. 2021 Dec;236:102874. doi: 10.1016/j.autneu.2021.102874. Epub 2021 Sep 11. Auton Neurosci. 2021. PMID: 34536906 Free PMC article. - The Role of ATP and Purinergic Receptors in Taste Signaling.
Kinnamon S, Finger T. Kinnamon S, et al. Handb Exp Pharmacol. 2022;275:91-107. doi: 10.1007/164_2021_518. Handb Exp Pharmacol. 2022. PMID: 34435233 Free PMC article. Review. - Taste transduction and channel synapses in taste buds.
Taruno A, Nomura K, Kusakizako T, Ma Z, Nureki O, Foskett JK. Taruno A, et al. Pflugers Arch. 2021 Jan;473(1):3-13. doi: 10.1007/s00424-020-02464-4. Epub 2020 Sep 16. Pflugers Arch. 2021. PMID: 32936320 Free PMC article. Review.
References
- Bader, P., and R. Weingart. 2004. Conductive and kinetic properties of connexin45 hemichannels expressed in transfected HeLa cells. J. Membr. Biol. 199:143–154. - PubMed
- Bao, L., S. Locovei, and G. Dahl. 2004. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 572:65–68. - PubMed
- Barbe, M.T., H. Monyer, and R. Bruzzone. 2006. Cell-cell communication beyond connexins: the pannexin channels. Physiology (Bethesda). 21:103–114. - PubMed
- Baryshnikov, S.G., O.A. Rogachevskaja, and S.S. Kolesnikov. 2003. Calcium signaling mediated by P2Y receptors in mouse taste cells. J. Neurophysiol. 90:3283–3294. - PubMed
- Braet, K., S. Aspeslagh, W. Vandamme, K. Willecke, P.E. Martin, W.H. Evans, and L. Leybaert. 2003. Pharmacological sensitivity of ATP release triggered by photoliberation of inositol-1,4,5-trisphosphate and zero extracellular calcium in brain endothelial cells. J. Cell. Physiol. 197:205–213. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous