A role for leukocyte-endothelial adhesion mechanisms in epilepsy - PubMed (original) (raw)
. 2008 Dec;14(12):1377-83.
doi: 10.1038/nm.1878. Epub 2008 Nov 23.
Graciela Navarro Mora, Marianna Martinello, Barbara Rossi, Flavia Merigo, Linda Ottoboni, Simona Bach, Stefano Angiari, Donatella Benati, Asmaa Chakir, Lara Zanetti, Federica Schio, Antonio Osculati, Pasquina Marzola, Elena Nicolato, Jonathon W Homeister, Lijun Xia, John B Lowe, Rodger P McEver, Francesco Osculati, Andrea Sbarbati, Eugene C Butcher, Gabriela Constantin
- PMID: 19029985
- PMCID: PMC2710311
- DOI: 10.1038/nm.1878
A role for leukocyte-endothelial adhesion mechanisms in epilepsy
Paolo F Fabene et al. Nat Med. 2008 Dec.
Abstract
The mechanisms involved in the pathogenesis of epilepsy, a chronic neurological disorder that affects approximately one percent of the world population, are not well understood. Using a mouse model of epilepsy, we show that seizures induce elevated expression of vascular cell adhesion molecules and enhanced leukocyte rolling and arrest in brain vessels mediated by the leukocyte mucin P-selectin glycoprotein ligand-1 (PSGL-1, encoded by Selplg) and leukocyte integrins alpha(4)beta(1) and alpha(L)beta(2). Inhibition of leukocyte-vascular interactions, either with blocking antibodies or by genetically interfering with PSGL-1 function in mice, markedly reduced seizures. Treatment with blocking antibodies after acute seizures prevented the development of epilepsy. Neutrophil depletion also inhibited acute seizure induction and chronic spontaneous recurrent seizures. Blood-brain barrier (BBB) leakage, which is known to enhance neuronal excitability, was induced by acute seizure activity but was prevented by blockade of leukocyte-vascular adhesion, suggesting a pathogenetic link between leukocyte-vascular interactions, BBB damage and seizure generation. Consistent with the potential leukocyte involvement in epilepsy in humans, leukocytes were more abundant in brains of individuals with epilepsy than in controls. Our results suggest leukocyte-endothelial interaction as a potential target for the prevention and treatment of epilepsy.
Figures
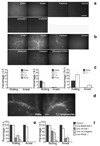
Figure 1. Alpha4 integrin, VCAM-1 and PSGL-1 dependence of PMN and TH1 cell interactions with brain endothelium after SE
a, b. Expression of adhesion molecules in cortical vessels was evaluated by in vivo staining and fluorescence microscopy before (Baseline) (a) and 6 h after the onset of seizure activity induced by pilocarpine (b). Isotype-matched mAb served as a control. Suppression of SE was achieved by administration of 3 mg/Kg Diazepam i.p. 20 min before pilocarpine injection (Diazepam) (b). Scale bar: 200 µm. c, e, f. The frequency of rolling interactions or sticking behavior of fluorescently labeled PMNs and lymphocyte subpopulations in cortical venules was studied before pilocarpine injection (0 h) and at 6 h and 24 h after SE onset by in situ videomicroscopy. The percentage of total cells shown on Y axis was calculated as described in Supplementary methods. The number of venules and animals per group analyzed are provided in Supplementary Table 1b, c. Mean ± SEM are shown. PLNs, freshly isolated peripheral lymph node cells (resting lymphocytes). d. Adherent PMNs 6 h post-SE and TH1 cells at 24 h post SE are shown in cerebral vessels (arrows). Scale bar: 100 µm. e, f. Anti-adhesion molecule mAbs were used to block leukocyte or endothelial adhesion molecules at 6 h after SE as described at Supplementary methods (Intravital microscopy subsection). PMNs are shown in e and TH1 cells are shown in f. Groups were compared with control using Kruskall-Wallis test followed by Bonferoni correction of P. **P<0.01; *P<0.001.
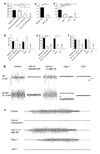
Figure 2. Effect of blockade or deficiency in leukocyte adhesion mechanisms on convulsions and seizures
In a-c visual monitoring of mean number of daily spontaneous convulsions (SC) per mouse was performed from day 5–20 after pilocarpine administration. Pilocarpine treated WT mice received no treatment (“epileptic mice”), or were injected i.p. either 1 h after SE-onset with 400 µg α4 integrin-specific mAb or with 150 µg VCAM-1-specific mAb, to model therapy; or 2 h prior to pilocarpine injection with 400 µg α4 integrin-specific mAb (pre-treatment regimen). In both instances treated mice also received antibody (200 µg anti-α4 or control mAb and 150 µg anti-VCAM-1) every other day for 20 days. An isotype-matched control antibody was used as control (a). 10–12 animals/group were monitored for SC for 6 h/day between day 5 and 20 after SE. One representative experiment from a series of 4 (a), 2 (b) and 3 (c) with similar results is shown. In d–f EEGs for each animal were acquired 24 h/day from day 0–20 after pilocarpine administration. Behaviorally, SRS were characterized in our mice by head nodding, forelimb clonus, rearing, and falling. d, days, s, seconds (g) Twenty-sec EEG recordings early after initial seizures and at the end of the second h are shown. Theta activity was occasionally observed in _Psgl-1_−/− animals (asterisk, fourth column, second line). (h) Recordings of representative SRS are shown. For d–h data are representative from one experiment with 3 mice/group from two experiments with similar results. ***P<0.001.
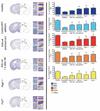
Figure 3. Effect of blockade or deficiency in leukocyte adhesion mechanisms on CNS histopathology
Epilepsy-induced structural changes and effects on neuronal cell density alterations were analyzed at 30 days after SE in healthy animals and in mice injected with pilocarpine: control (C57Bl/6, WT) mice, anti-α4 treated animals and _Psgl-1_−/− and _Fut7_−/− mice. For quantitative evaluation, the number of Nissl stained cell bodies was counted in four sections/animal (3 animals/group) using regions of interest (ROI) sampling. The ROI was delimited by a rectangular frame which was placed within the sampled cortical and hippocampal areas, using upper-left exclusion lines. In the somatosensory (SS) cortex, the rectangular frame was placed vertically throughout the cortical thickness, superficial (II-III) (SS1) and deep (V-VI) layers (SS2) of the cortex. See also the Supplementary Methods for details. Mean ± SEM are shown for stereological counts. Statistical comparison of anti-α4-treated groups or _Psgl-1_−/− and _Fut7_−/− groups with Control/WT epileptic condition was performed using a two-tail _t_-test. *P<0.04. CA, corpus ammonis. Scale bar corresponds to 30 µm.
Figure 4. Inhibition or deficiency of leukocyte adhesion pathways maintains the integrity of the BBB
(a) Evans Blue (EB) extravasation was evaluated 18–24 h after pilocarpine injection. In WT (control) mice, EB leakage (asterisks) was observed macroscopically in brains, after removal of the meninges, in the parietal cortex and in particular in the territories relative to the medial cerebral artery (black asterisks) in the superior and lateral views; in the pituitary gland pedunculus (where the BBB is more permeable) in the inferior view; and, finally, in the choroid plexus of the lateral ventricles and in the fourth ventricle as in the sagittal view. WT animals injected only with methylscopolamine did not show any leakage (data not shown). Anti-α4 pretreatment as well as PSGL1- or FucT-VII deficiency (_Psgl-1_−/− and _Fut7_−/− mice) prevented BBB leakage. Representative brains are shown from one of 2 experiments, 3 mice/condition, with similar results. Scale bar: 4 mm. (b) BBB alterations post-SE were studied in control (C57Bl/6/, WT mice), and anti-α4 treated animals and in _Psgl-1_−/− and _Fut_−/− mice at 24 h after pilocarpine injection. BBB permeability was studied by the mean of intravenously administered Magnevist® (Nmethylglucamine salt of gadolinium complex of diethylenetriamine pentaacetic acid) a paramagnetic iron oxide contrast agent in MRI. Pseudo-color maps evidencing contrast agent spreading are shown. Representative brains are shown from one of two experiments (3mice/condition) with similar results. Scale bar: 2 mm.
Comment in
- Brain inflammation initiates seizures.
Kleen JK, Holmes GL. Kleen JK, et al. Nat Med. 2008 Dec;14(12):1309-10. doi: 10.1038/nm1208-1309. Nat Med. 2008. PMID: 19057551 No abstract available. - Leukocyte-endothelial adhesion mechanisms in epilepsy: cheers and jeers.
Vezzani A, Janigro D. Vezzani A, et al. Epilepsy Curr. 2009 Jul-Aug;9(4):118-21. doi: 10.1111/j.1535-7511.2009.01312.x. Epilepsy Curr. 2009. PMID: 19693331 Free PMC article. No abstract available. - Breaching the barrier and inflaming epilepsy research.
Sills GJ, Solomon T. Sills GJ, et al. Epilepsy Curr. 2009 Sep-Oct;9(5):148-50. doi: 10.1111/j.1535-7511.2009.01324.x. Epilepsy Curr. 2009. PMID: 19826510 Free PMC article. No abstract available.
Similar articles
- Macrophage migration inhibitory factor increases leukocyte-endothelial interactions in human endothelial cells via promotion of expression of adhesion molecules.
Cheng Q, McKeown SJ, Santos L, Santiago FS, Khachigian LM, Morand EF, Hickey MJ. Cheng Q, et al. J Immunol. 2010 Jul 15;185(2):1238-47. doi: 10.4049/jimmunol.0904104. Epub 2010 Jun 16. J Immunol. 2010. PMID: 20554956 - L- and P-selectins collaborate to support leukocyte rolling in vivo when high-affinity P-selectin-P-selectin glycoprotein ligand-1 interaction is inhibited.
Ridger VC, Hellewell PG, Norman KE. Ridger VC, et al. Am J Pathol. 2005 Mar;166(3):945-52. doi: 10.1016/S0002-9440(10)62314-0. Am J Pathol. 2005. PMID: 15743805 Free PMC article. - P-Selectin Glycoprotein Ligand-1 Deficiency Protects Against Aortic Aneurysm Formation Induced by DOCA Plus Salt.
Wu X, Liu X, Yang H, Chen Q, Zhang N, Li Y, Du X, Liu X, Jiang X, Jiang Y, Zhou Z, Yang Z. Wu X, et al. Cardiovasc Drugs Ther. 2022 Feb;36(1):31-44. doi: 10.1007/s10557-020-07135-1. Epub 2021 Jan 11. Cardiovasc Drugs Ther. 2022. PMID: 33432452 - Are you in or out? Leukocyte, ion, and neurotransmitter permeability across the epileptic blood-brain barrier.
Janigro D. Janigro D. Epilepsia. 2012 Jun;53 Suppl 1(0 1):26-34. doi: 10.1111/j.1528-1167.2012.03472.x. Epilepsia. 2012. PMID: 22612806 Free PMC article. Review. - Therapeutic strategies in autoimmune diseases by interfering with leukocyte endothelium interaction.
Rychly J, Nebe B. Rychly J, et al. Curr Pharm Des. 2006;12(29):3799-806. doi: 10.2174/138161206778559696. Curr Pharm Des. 2006. PMID: 17073678 Review.
Cited by
- Blood-brain barrier, bulk flow, and interstitial clearance in epilepsy.
Marchi N, Banjara M, Janigro D. Marchi N, et al. J Neurosci Methods. 2016 Feb 15;260:118-24. doi: 10.1016/j.jneumeth.2015.06.011. Epub 2015 Jun 18. J Neurosci Methods. 2016. PMID: 26093166 Free PMC article. Review. - Inflammation and epilepsy: the foundations for a new therapeutic approach in epilepsy?
Walker L, Sills GJ. Walker L, et al. Epilepsy Curr. 2012 Jan;12(1):8-12. doi: 10.5698/1535-7511-12.1.8. Epilepsy Curr. 2012. PMID: 22368518 Free PMC article. - Protein expression profiling of inflammatory mediators in human temporal lobe epilepsy reveals co-activation of multiple chemokines and cytokines.
Kan AA, de Jager W, de Wit M, Heijnen C, van Zuiden M, Ferrier C, van Rijen P, Gosselaar P, Hessel E, van Nieuwenhuizen O, de Graan PN. Kan AA, et al. J Neuroinflammation. 2012 Aug 30;9:207. doi: 10.1186/1742-2094-9-207. J Neuroinflammation. 2012. PMID: 22935090 Free PMC article. - Blood-brain barrier dysfunction in epileptogenesis of the temporal lobe.
Weissberg I, Reichert A, Heinemann U, Friedman A. Weissberg I, et al. Epilepsy Res Treat. 2011;2011:143908. doi: 10.1155/2011/143908. Epub 2011 Jun 7. Epilepsy Res Treat. 2011. PMID: 22937228 Free PMC article. - Key factors in the discovery and development of new antiepileptic drugs.
Bialer M, White HS. Bialer M, et al. Nat Rev Drug Discov. 2010 Jan;9(1):68-82. doi: 10.1038/nrd2997. Nat Rev Drug Discov. 2010. PMID: 20043029 Review.
References
- Strzelczyk A, Reese JP, Dodel R, Hamer HM. Cost of epilepsy: a systematic review. Pharmacoeconomics. 2008;26:463–476. - PubMed
- Holmes GL. Seizure-induced neuronal injury: animal data. Neurology. 2002;59:S3–S6. - PubMed
- Duncan JS. Seizure-induced neuronal injury: human data. Neurology. 2002;59:S15–S20. - PubMed
- Vezzani A, Granata T. Brain inflammation in epilepsy: experimental and clinical evidence. Epilepsia. 2005;46:1724–1743. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 CA071932/CA/NCI NIH HHS/United States
- R01 AI072618/AI/NIAID NIH HHS/United States
- P01 HL085607/HL/NHLBI NIH HHS/United States
- R01 HL090823/HL/NHLBI NIH HHS/United States
- P01 CA071932-11A2/CA/NCI NIH HHS/United States
- P01 HL085607-01/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases