Exploring the full spectrum of macrophage activation - PubMed (original) (raw)
Review
Exploring the full spectrum of macrophage activation
David M Mosser et al. Nat Rev Immunol. 2008 Dec.
Erratum in
- Nat Rev Immunol.2010 Jun;10(6):460
Abstract
Macrophages display remarkable plasticity and can change their physiology in response to environmental cues. These changes can give rise to different populations of cells with distinct functions. In this Review we suggest a new grouping of macrophage populations based on three different homeostatic activities - host defence, wound healing and immune regulation. We propose that similarly to primary colours, these three basic macrophage populations can blend into various other 'shades' of activation. We characterize each population and provide examples of macrophages from specific disease states that have the characteristics of one or more of these populations.
Figures
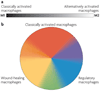
Figure 1. Colour wheel of macrophage activation
a | A monochromatic depiction of the previous nomenclature showing the linear scale of the two macrophage designations, M1 and M2. b | The three populations of macrophages that are discussed in this article are arranged according to the three primary colours, with red designating classically activated macrophages, yellow designating wound-healing macrophages and blue designating regulatory macrophages. Secondary colours, such as green, may represent tumour-associated macrophages, which have many characteristics of regulatory macrophages but also share some characteristics of wound-healing macrophages. In obese individuals, wound-healing macrophages may transit towards a classically activated-macrophage phenotype.
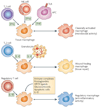
Figure 2. Monocyte heterogeneity
Monocytes originate in the bone marrow from a common haematopoietic stem cell (HSC). They undergo differentiation steps during which they commit to the myeloid and then to a monocyte lineage. In response to macrophage colony-stimulating factor, they divide and differentiate into monoblasts and then pro-monocytes before becoming monocytes, which exit the bone marrow and enter the bloodstream. In mice, there is evidence of two distinct monocyte populations in the blood that have different phenotypes and biochemical signatures. GR1+CX3CR1low (CX3C-chemokine receptor 1) monocytes rapidly exit the blood, and for this reason they are referred to as ‘inflammatory’ monocytes. GR1− monocytes have been termed ‘resident’ monocytes to differentiate them from the other population. It remains unknown whether inflammatory monocytes mature into resident monocytes in the blood or whether these two cells represent distinct monocyte populations. Human monocytes can also be divided into two populations, but the designations inflammatory and resident do not apply to these populations. Monocytes migrate to different tissues, where they replenish tissue-specific macrophages. CNS, central nervous system; GM-CFU, granulocyte/macrophage colony-forming unit; M-CFU, macrophage colony-forming unit.
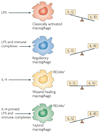
Figure 3. Cytokines produced by immune cells can give rise to macrophages with distinct physiologies
Classically activated macrophages arise in response to interferon-γ (IFNγ), which can be produced during an adaptive immune response by T helper 1 (TH1) cells or CD8+ T cells (not shown) or during an innate immune response by natural killer (NK) cells, and tumour-necrosis factor (TNF), which is produced by antigen-presenting cells (APCs). Wound-healing (alternatively activated) macrophages arise in response to interleukin-4 (IL-4), which can be produced during an adaptive immune response by TH2 cells or during an innate immune response by granulocytes. Regulatory macrophages are generated in response to various stimuli, including immune complexes, prostaglandins, G-protein coupled receptor (GPCR) ligands, glucocorticoids, apoptotic cells or IL-10. Each of these three populations has a distinct physiology. Classically activated macrophages have microbicidal activity, whereas regulatory macrophages produce high levels of IL-10 to suppress immune responses. Wound-healing macrophages are similar to the previously described alternatively activated macrophages and have a role in tissue repair. TLR, Toll-like receptor.
Figure 4. Interactions between macrophage and T cells
a | Interferon-γ (IFNγ) produced by T helper 1 (TH1) cells or CD8+ T cells, along with tumour-necrosis factor (TNF) from antigen-presenting cells, can give rise to classically activated macrophages, which secrete interleukin-1 (IL-1), IL-6 and IL-23. These cytokines can give rise to TH17 cells, which can contribute to autoimmune responses. Classically activated macrophages also produce IL-12 to promote the differentiation of TH1 cells, but they can also produce IL-27, which inhibits various immune responses and negatively regulates TH1 and TH2 cells. b | IL-10 produced by regulatory T cells can give rise to a population of regulatory macrophages, which act as antigen-presenting cells, produce IL-10 and can induce the expansion of TH2 cells. There is controversy about whether some regulatory macrophages can also promote the development of regulatory T cells. IL-4 and/or IL-13 produced by TH2 cells can promote the development of wound-healing macrophages, but these macrophages are poor antigen-presenting cells and may even inhibit T-cell proliferation.
Figure 5. The plasticity of activated macrophages
Classically activated macrophages produce high levels of interleukin-12 (IL-12) and modest levels of IL-10. By contrast, regulatory macrophages produce high levels of IL-10 and low levels of IL-12. Macrophages treated with IL-4 (that is, wound-healing macrophages) produce low levels of these cytokines, but express resistin-like molecule-α (RELMα) intracellularly, a marker that is not expressed by the other macrophage populations. Treatment of IL-4-primed macrophages with lipopolysaccharide (LPS) and immune complexes results in a hybrid phenotype in which the cells continue to express RELMα (similarly to wound-healing macrophages) but also produce high levels of IL-10 (similarly to regulatory macrophages).
Similar articles
- Diversity, Mechanisms, and Significance of Macrophage Plasticity.
Locati M, Curtale G, Mantovani A. Locati M, et al. Annu Rev Pathol. 2020 Jan 24;15:123-147. doi: 10.1146/annurev-pathmechdis-012418-012718. Epub 2019 Sep 17. Annu Rev Pathol. 2020. PMID: 31530089 Free PMC article. Review. - Wound healing: the role of the macrophage and other immune cells.
DiPietro LA. DiPietro LA. Shock. 1995 Oct;4(4):233-40. Shock. 1995. PMID: 8564549 Review. - From inflammation to wound healing: using a simple model to understand the functional versatility of murine macrophages.
Childs LM, Paskow M, Morris SM Jr, Hesse M, Strogatz S. Childs LM, et al. Bull Math Biol. 2011 Nov;73(11):2575-604. doi: 10.1007/s11538-011-9637-5. Epub 2011 Feb 23. Bull Math Biol. 2011. PMID: 21347813 Free PMC article. - Amnion-derived cellular cytokine solution promotes macrophage activity.
Uberti MG, Lufkin AE, Pierpont YN, Ko F, Smith CA, Robson MC, Payne WG. Uberti MG, et al. Ann Plast Surg. 2011 May;66(5):575-80. doi: 10.1097/SAP.0b013e318212f1d0. Ann Plast Surg. 2011. PMID: 21451377 - Macrophage Motility in Wound Healing Is Regulated by HIF-1α via S1P Signaling.
Hutami IR, Izawa T, Khurel-Ochir T, Sakamaki T, Iwasa A, Tanaka E. Hutami IR, et al. Int J Mol Sci. 2021 Aug 20;22(16):8992. doi: 10.3390/ijms22168992. Int J Mol Sci. 2021. PMID: 34445695 Free PMC article. Review.
Cited by
- Practical immunomodulatory landscape of glioblastoma multiforme (GBM) therapy.
Norollahi SE, Yousefi B, Nejatifar F, Yousefzadeh-Chabok S, Rashidy-Pour A, Samadani AA. Norollahi SE, et al. J Egypt Natl Canc Inst. 2024 Oct 28;36(1):33. doi: 10.1186/s43046-024-00240-4. J Egypt Natl Canc Inst. 2024. PMID: 39465481 Review. - Intake of korean red ginseng extract and saponin enhances the protection conferred by vaccination with inactivated influenza a virus.
Xu ML, Kim HJ, Choi YR, Kim HJ. Xu ML, et al. J Ginseng Res. 2012 Oct;36(4):396-402. doi: 10.5142/jgr.2012.36.4.396. J Ginseng Res. 2012. PMID: 23717142 Free PMC article. - PACAP attenuates NMDA-induced retinal damage in association with modulation of the microglia/macrophage status into an acquired deactivation subtype.
Wada Y, Nakamachi T, Endo K, Seki T, Ohtaki H, Tsuchikawa D, Hori M, Tsuchida M, Yoshikawa A, Matkovits A, Kagami N, Imai N, Fujisaka S, Usui I, Tobe K, Koide R, Takahashi H, Shioda S. Wada Y, et al. J Mol Neurosci. 2013 Oct;51(2):493-502. doi: 10.1007/s12031-013-0017-5. Epub 2013 May 30. J Mol Neurosci. 2013. PMID: 23720065 - Time-resolved systems immunology reveals a late juncture linked to fatal COVID-19.
Liu C, Martins AJ, Lau WW, Rachmaninoff N, Chen J, Imberti L, Mostaghimi D, Fink DL, Burbelo PD, Dobbs K, Delmonte OM, Bansal N, Failla L, Sottini A, Quiros-Roldan E, Han KL, Sellers BA, Cheung F, Sparks R, Chun TW, Moir S, Lionakis MS; NIAID COVID Consortium; COVID Clinicians; Rossi C, Su HC, Kuhns DB, Cohen JI, Notarangelo LD, Tsang JS. Liu C, et al. Cell. 2021 Apr 1;184(7):1836-1857.e22. doi: 10.1016/j.cell.2021.02.018. Epub 2021 Feb 10. Cell. 2021. PMID: 33713619 Free PMC article. - Epoxyeicosatrienoic acids regulate macrophage polarization and prevent LPS-induced cardiac dysfunction.
Dai M, Wu L, He Z, Zhang S, Chen C, Xu X, Wang P, Gruzdev A, Zeldin DC, Wang DW. Dai M, et al. J Cell Physiol. 2015 Sep;230(9):2108-19. doi: 10.1002/jcp.24939. J Cell Physiol. 2015. PMID: 25626689 Free PMC article.
References
- Nathan C. Metchnikoff’s legacy in 2008. Nature Immunol. 2008;9:695–698. - PubMed
- Chen CJ, et al. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nature Med. 2007;13:851–856. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI49388/AI/NIAID NIH HHS/United States
- R01 AI049383/AI/NIAID NIH HHS/United States
- R01 AI055576/AI/NIAID NIH HHS/United States
- R01 AI049388/AI/NIAID NIH HHS/United States
- R21 AI055576/AI/NIAID NIH HHS/United States
- R01 AI049383-07/AI/NIAID NIH HHS/United States
- AI55576/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources