RNA-based gene duplication: mechanistic and evolutionary insights - PubMed (original) (raw)
Review
RNA-based gene duplication: mechanistic and evolutionary insights
Henrik Kaessmann et al. Nat Rev Genet. 2009 Jan.
Abstract
Gene copies that stem from the mRNAs of parental source genes have long been viewed as evolutionary dead-ends with little biological relevance. Here we review a range of recent studies that have unveiled a significant number of functional retroposed gene copies in both mammalian and some non-mammalian genomes. These studies have not only revealed previously unknown mechanisms for the emergence of new genes and their functions but have also provided fascinating general insights into molecular and evolutionary processes that have shaped genomes. For example, analyses of chromosomal gene movement patterns via RNA-based gene duplication have shed fresh light on the evolutionary origin and biology of our sex chromosomes.
Figures
Figure 1. Mechanism of gene retroposition
(A) Gene retroposition is initiated with the transcription of a parental gene by RNA polymerase II and (B) further processing of its RNA (splicing and polyadenylation), which produces a mature mRNA. (C) Gene retroposition is mediated by the L1 endonuclease domain (pink hourglass) that creates a first nick (yellow star) at the genomic site of insertion at the TTAAAA target sequence. (D) This nick enables the priming of the reverse transcription (by the L1 reverse transcription domain; pink oval shape), which uses the parental mRNA as template. (E) Second strand nick generation (precise mechanism not known). (F) Second DNA strand synthesis (precise mechanism not known). (G) Complementary DNA synthesis in overhang regions created by the two nicks, which creates a duplication of the sequence flanking the target sequence, which is one of the molecular signatures of gene retroposition, in addition to the lack of introns and the presence of a poly-A tail (the direct repeats and the poly-A tail degenerate upon time and are therefore usually only detectable in recent retrocopies). The illustration is based on findings described in references -.
Figure 2. Source of retrogene promoters
The figure illustrates various scenarios that lead to the transcription of retroposed gene copies. (A) Retrocopies may insert into intronic sequences of host genes. The evolution and/or presence of splicing signals enable these copies to be integrated into new splice variants of their host gene. Depending on the localization of these new splice sites, these variants result in either non-coding fusion transcripts (where the entire open reading frame derives from the retrocopy) or coding sequence fusions (the coding region of the retrocopy is fused to that of the host gene). (B) The insertion of retrocopies into actively transcribed regions with an open chromatin structure facilitates their transcription, due to the increased accessibility for the transcriptional machinery. The presence of enhancer elements from neighboring genes and weak transcription promoting sequences (not previously associated with genes) can further strengthen their transcriptional activity. (C) Recruitment of distant promoters in the genomic neighborhood via the acquisition of a new untranslated exon/intron structure. (D) Recruitment of promoters from retrotransposons or CpG proto-promoters. (E) Inheritance of parental promoters through alternative transcriptional start site usage of the parental gene. (F) De novo promoter evolution in the 5′ flanking region of the insertion site by single nucleotide substitutions.
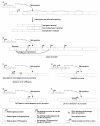
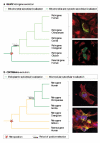
Figure 3. Subcellular adaptation of proteins encoded by new duplicate genes
(A) Illustration of 2 scenarios for the evolution of duplicated genes (red and green) and their products. Each gene and its encoded protein are represented with one color. Distinct protein shapes indicate distinct functions. Three different protein localizations (cytosolic, endoplasmic reticulum, or secreted proteins) are indicated in a schematic cell. Positively selected substitutions responsible for subcellular changes or changes in protein function are indicated (arrows). See main text for references and further details. (B) Adaptive evolution of two primate specific retrogenes (GLUD2 left, CDC14Bretro right). Phylogenetic trees indicate retroduplication events. Periods of adaptive evolution and reconstructed subcellular localizations are indicated. Microscopy images display representative subcellular phenotypes for the indicated branches. Markers on the left: protein localization (green), nuclear DNA (blue), and microtubules (red). Yellow signals indicate an overlap of the protein with microtubules. Markers on the right: protein localization (green) and mitochondria (red).
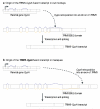
Figure 4. Origin of TRIM5-CypA gene fusions in macques and owl monkeys
(A) Retroposition of CypA into an intron of the TRIM5 gene from macaques and the resulting fusion gene is shown (similar to the process displayed in Fig. 2A). (B) An independent retroposition of CypA into the UTR of TRIM5 in owl monkeys is shown, also resulting in a new TRIM5-CypA fusion gene. Please refer to Fig. 2 for the colour code and to the main text for details.
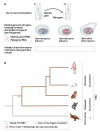
Figure 5. Retrogenes, MSCI, and the emergence of mammalian sex chromosomes
(A, upper part) Illustration of the retroposition of an X-linked parental gene to an autosome. (A, lower part) Illustration of the expression of X-linked parental genes and their autosomal retrogene copies before (in spermatogonial cells), during (spermatocytes), and after (spermatids) the process of meiotic sex chromosome inactivation (MSCI). (B) The evolutionary onset for the selectively driven out of X retroduplication process and MSCI, as well as the inferred origin of therian (eutherians/placental mammals and metatherians/marsupials) sex chromosomes. See main text for further explanations.
References
- Long M, Betran E, Thornton K, Wang W. The origin of new genes: Glimpses from the young and old. Nature Reviews Genetics. 2003;4:865–875. - PubMed
- Ohno S. Evolution by Gene Duplication. Springer Verlag; Berlin: 1970.
- Wolfe KH, Li WH. Molecular evolution meets the genomics revolution. Nat Genet. 2003;33(Suppl):255–65. - PubMed
- Prince VE, Pickett FB. Splitting pairs: the diverging fates of duplicated genes. Nat Rev Genet. 2002;3:827–37. - PubMed
- Lynch M. The origins of genome architecture. Sinauer Associates; Sunderland, USA: 2007.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources