Molecular organization of Gram-negative peptidoglycan - PubMed (original) (raw)
Molecular organization of Gram-negative peptidoglycan
Lu Gan et al. Proc Natl Acad Sci U S A. 2008.
Abstract
The stress-bearing component of the bacterial cell wall--a multi-gigadalton bag-like molecule called the sacculus--is synthesized from peptidoglycan. Whereas the chemical composition and the 3-dimensional structure of the peptidoglycan subunit (in at least one conformation) are known, the architecture of the assembled sacculus is not. Four decades' worth of biochemical and electron microscopy experiments have resulted in two leading 3-D peptidoglycan models: "Layered" and "Scaffold", in which the glycan strands are parallel and perpendicular to the cell surface, respectively. Here we resolved the basic architecture of purified, frozen-hydrated sacculi through electron cryotomography. In the Gram-negative sacculus, a single layer of glycans lie parallel to the cell surface, roughly perpendicular to the long axis of the cell, encircling the cell in a disorganized hoop-like fashion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Models of peptidoglycan organization. The Layered (A), Scaffold (B), and Disorganized Layered (C) models are viewed obliquely to the sacculus surface and toward the cell pole. Disaccharides (green pills) are approximately 1-nm long and the stretched peptide crosslinks (blue sticks) are approximately 4 nm long. The length distribution of the glycan strands (3-mers to 14-mers) approximates the resolvable part of HPLC chromatograms of purified, hydrolyzed sacculi (10), and the crosslinkage is kept less than the experimentally determined maximum of 50% (21). It is not known whether glycans run parallel or antiparallel to each other.
Fig. 2.
Comparison of in situ and in vitro sacculi. (A) Twenty-five-nm thick Z-slice through the 3-D reconstruction of a C. crescentus CB15N cell. (B) 10-nm thick Z-slice through the 3-D reconstruction of a purified sacculus. Both panels are at the same scale. Abbreviations: (SL) S-layer; (OM) outer membrane; (PG) peptidoglycan layer, that is, the sacculus; (IM) inner membrane; (r) ribosome-like particle; (St) stalk; (Au) gold fiducial; (gr) granule; (gl) glycan strand; (SW) side wall. (Inset) Close-up of sacculus showing glycan strands. Note that the highly flexible sacculus has flattened because of the surface tension of the thin aqueous film, so that it appears wider than the intact cell. The cell tomogram is from reference (16). (C) Enlarged view (4-nm thick slice) showing the glycan strands in the sacculus side wall boxed in white in (B). (D) The same glycan strands as in (C), rotated approximately 90° around the vertical axis.
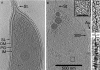
Fig. 3.
Tomographic slices of E. coli sacculi. Ten-nm thick Z-slices of E. coli strain MG1655 (A) and XL-10 sacculi (B). Abbreviations are the same as in Fig. 2 except for: (w) wrinkle. The double-headed arrow denotes the saccular polar axis. (Insets) Four-fold enlarged views of the boxed regions. Note that the direction of the strand densities is approximately perpendicular to the saccular polar axis. Compared to C. crescentus, E. coli sacculi images had poorer contrast, which may be a result of the ice surrounding E. coli sacculi being at least 20 nm thicker.
Fig. 4.
Details of peptidoglycan organization. (A) 30-nm thick Z-slice of two overlapping C. crescentus CB15N sacculi, outlined by green or violet dotted lines. Abbreviations are the same as in Figs. 3 and 3 except for: (cf) carbon support film. (Insets) 30-nm thick cross-sections cut through the yellow line in the main panel. The yellow dotted line follows the “upper” and “lower” halves of the peptidoglycan. (B) Enlarged view (4-nm thick slice) of the sacculus side wall boxed in (A). (C) Image (B) after 3-D median filtration; one putative glycan strand is highlighted in blue. The green-shaded region demarcates the peptidoglycan. (D) Isosurface rendering the volume shown in (C). The putative glycan strand in (D) is boxed in blue. The noise densities outside of the sacculus are colored gray for clarity. (Inset) Superposition of glycan 9-mer atomic model in the blue-boxed density, shown for a sense of scale.
Similar articles
- Thickness and elasticity of gram-negative murein sacculi measured by atomic force microscopy.
Yao X, Jericho M, Pink D, Beveridge T. Yao X, et al. J Bacteriol. 1999 Nov;181(22):6865-75. doi: 10.1128/JB.181.22.6865-6875.1999. J Bacteriol. 1999. PMID: 10559150 Free PMC article. - Architecture and assembly of the Gram-positive cell wall.
Beeby M, Gumbart JC, Roux B, Jensen GJ. Beeby M, et al. Mol Microbiol. 2013 May;88(4):664-72. doi: 10.1111/mmi.12203. Epub 2013 Apr 22. Mol Microbiol. 2013. PMID: 23600697 Free PMC article. - Peptidoglycan structure and architecture.
Vollmer W, Blanot D, de Pedro MA. Vollmer W, et al. FEMS Microbiol Rev. 2008 Mar;32(2):149-67. doi: 10.1111/j.1574-6976.2007.00094.x. Epub 2008 Jan 8. FEMS Microbiol Rev. 2008. PMID: 18194336 Review. - Orientation of the peptidoglycan chains in the sacculus of Escherichia coli.
Koch AL. Koch AL. Res Microbiol. 1998 Nov-Dec;149(10):689-701. doi: 10.1016/s0923-2508(99)80016-3. Res Microbiol. 1998. PMID: 9921576 Review. - Architecture of peptidoglycan: more data and more models.
Vollmer W, Seligman SJ. Vollmer W, et al. Trends Microbiol. 2010 Feb;18(2):59-66. doi: 10.1016/j.tim.2009.12.004. Epub 2010 Jan 8. Trends Microbiol. 2010. PMID: 20060721 Review.
Cited by
- Structural constraints and dynamics of bacterial cell wall architecture.
de Pedro MA, Cava F. de Pedro MA, et al. Front Microbiol. 2015 May 8;6:449. doi: 10.3389/fmicb.2015.00449. eCollection 2015. Front Microbiol. 2015. PMID: 26005443 Free PMC article. Review. - Mechanical consequences of cell-wall turnover in the elongation of a Gram-positive bacterium.
Misra G, Rojas ER, Gopinathan A, Huang KC. Misra G, et al. Biophys J. 2013 Jun 4;104(11):2342-52. doi: 10.1016/j.bpj.2013.04.047. Biophys J. 2013. PMID: 23746506 Free PMC article. - Physics of bacterial morphogenesis.
Sun SX, Jiang H. Sun SX, et al. Microbiol Mol Biol Rev. 2011 Dec;75(4):543-65. doi: 10.1128/MMBR.00006-11. Microbiol Mol Biol Rev. 2011. PMID: 22126993 Free PMC article. Review. - The peptidoglycan-associated protein NapA plays an important role in the envelope integrity and in the pathogenesis of the lyme disease spirochete.
Davis MM, Brock AM, DeHart TG, Boribong BP, Lee K, McClune ME, Chang Y, Cramer N, Liu J, Jones CN, Jutras BL. Davis MM, et al. PLoS Pathog. 2021 May 13;17(5):e1009546. doi: 10.1371/journal.ppat.1009546. eCollection 2021 May. PLoS Pathog. 2021. PMID: 33984073 Free PMC article. - Cryo-electron tomography elucidates the molecular architecture of Treponema pallidum, the syphilis spirochete.
Izard J, Renken C, Hsieh CE, Desrosiers DC, Dunham-Ems S, La Vake C, Gebhardt LL, Limberger RJ, Cox DL, Marko M, Radolf JD. Izard J, et al. J Bacteriol. 2009 Dec;191(24):7566-80. doi: 10.1128/JB.01031-09. Epub 2009 Oct 9. J Bacteriol. 2009. PMID: 19820083 Free PMC article.
References
- Formanek H, Formanek S. Specific staining for electron microscopy of murein sacculi of bacterial cell walls. Eur J Biochem. 1970;17:78–84. - PubMed
- Dietrich I, Formanek H, Fox F, Knapek E, Weyl R. Reduction of radiation-damage in an electron-microscope with a superconducting lens system. Nature. 1979;277:380–381. - PubMed
- De Petris S. Ultrastructure of the cell wall of Escherichia coli and chemical nature of its constituent layers. J Ultra Res. 1967;19:45–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 GM082545/GM/NIGMS NIH HHS/United States
- R01 AI067548/AI/NIAID NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R01 AI1067548/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources