Roles of IP3R and RyR Ca2+ channels in endoplasmic reticulum stress and beta-cell death - PubMed (original) (raw)
Roles of IP3R and RyR Ca2+ channels in endoplasmic reticulum stress and beta-cell death
Dan S Luciani et al. Diabetes. 2009 Feb.
Abstract
Objective: Endoplasmic reticulum (ER) stress has been implicated in the pathogenesis of diabetes, but the roles of specific ER Ca(2+) release channels in the ER stress-associated apoptosis pathway remain unknown. Here, we examined the effects of stimulating or inhibiting the ER-resident inositol trisphosphate receptors (IP(3)Rs) and the ryanodine receptors (RyRs) on the induction of beta-cell ER stress and apoptosis.
Research design and methods: Kinetics of beta-cell death were tracked by imaging propidium iodide incorporation and caspase-3 activity in real time. ER stress and apoptosis were assessed by Western blot. Mitochondrial membrane potential was monitored by flow cytometry. Cytosolic Ca(2+) was imaged using fura-2, and genetically encoded fluorescence resonance energy transfer (FRET)-based probes were used to measure Ca(2+) in ER and mitochondria.
Results: Neither RyR nor IP(3)R inhibition, alone or in combination, caused robust death within 24 h. In contrast, blocking sarco/endoplasmic reticulum ATPase (SERCA) pumps depleted ER Ca(2+) and induced marked phosphorylation of PKR-like ER kinase (PERK) and eukaryotic initiation factor-2alpha (eIF2alpha), C/EBP homologous protein (CHOP)-associated ER stress, caspase-3 activation, and death. Notably, ER stress following SERCA inhibition was attenuated by blocking IP(3)Rs and RyRs. Conversely, stimulation of ER Ca(2+) release channels accelerated thapsigargin-induced ER depletion and apoptosis. SERCA block also activated caspase-9 and induced perturbations of the mitochondrial membrane potential, resulting eventually in the loss of mitochondrial polarization.
Conclusions: This study demonstrates that the activity of ER Ca(2+) channels regulates the susceptibility of beta-cells to ER stress resulting from impaired SERCA function. Our results also suggest the involvement of mitochondria in beta-cell apoptosis associated with dysfunctional beta-cell ER Ca(2+) homeostasis and ER stress.
Figures
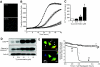
FIG. 1.
ER Ca2+ dynamics during acute SERCA inhibition and IP3R activation in MIN6 β-cells. A: Subcellular colocalization of mRFP targeted to the ER using the KDEL protein sequence and DIER cameleon in transfected MIN6 cells. B: A total of 1 μmol/l thapsigargin evoked a gradual depletion of luminal ER Ca2+. An average trace is shown in black (n = 14 cells), and profiles from individual cells are shown to illustrate the response heterogeneity. C: Repeatable and reversible lowering of ER luminal Ca2+ due to IP3R activation by successive carbachol (Cch) treatments as indicated. An average response is shown in the black trace (n = 9 cells) along with representative single cell traces. (Please see
http://dx.doi.org/10.2337/db07-1762
for a high-quality digital representation of this figure.)
FIG. 2.
Dose- and time-dependent effects of SERCA inhibition on CHOP expression, caspase-3 activation, and cell death. Cell death was assayed in real-time by propidium iodide incorporation in MIN6 cells. A: Images illustrating the progressive propidium iodide incorporation in a field of MIN6 cells exposed to 1 μmol/l thapsigargin (Tg). B: Representative time course of cell death in response to various concentrations of thapsigargin. ○, Control; ▪, 0.01 μmol/l thapsigargin; ▴, 0.1 μmol/l thapsigargin; •, 1 μmol/l thapsigargin. C: Dose dependence of the thapsigargin-induced MIN6 cell death, quantified as the area under the curves (IAUC) of the first 24 h of the propidium iodide incorporation profiles (n = 3). D: Induction of CHOP (∼31-kDa band) and cleaved caspase-3 (∼17- to 19-kDa band) in MIN6 cells cultured for 24 h in DMEM containing 25 mmol/l glucose and increasing concentrations of thapsigargin (n = 3). E: Representative real-time imaging of caspase-3 activation in living MIN6 cells using the MiCy-DEVD-mKO FRET probe. The loss of FRET/MiCy intensity ratio, observed in the cell marked as number one, between the time points marked (a) and (b), results from cleavage of the DEVD caspase-3 target sequence. The cells were imaged for a period of 5 h, during which caspase-3 was activated in 5 of 16 (31%) thapsigargin-treated cells and in 1 of 10 (10%) control cells. (Please see
http://dx.doi.org/10.2337/db07-1762
for a high-quality digital representation of this figure.)
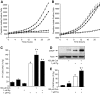
FIG. 3.
ER stress and caspase-3–dependent cell death is induced by blocking Ca2+ pumps but not ER Ca2+ release channels. A: MIN6 cell death was monitored over 24 h in response to inhibition of RyR (100 μmol/l ryanodine), IP3R (1 μmol/l xestospongin C), combined inhibition of RyR and IP3R, or inhibition of SERCA pumps (1 μmol/l thapsigargin). All inhibitors were applied in culture media containing 25 mmol/l glucose. Traces are representative of six independent experiments. ○, control; ▵, 100 μmol/l ryanodine; ▴, 1 μmol/l xestospongin C;  , 100 μmol/l ryanodine + 1 μmol/l xestaspongin C; •, 1 μmol/l thapsigargin. B: MIN6 cells were cultured as indicated and probed for markers of ER stress and apoptosis, as in Fig. 2. CHOP expression was examined and quantified at both low and high glucose (n = 4–10). C: Cleaved caspase-3 expression examined and quantified at both low and high glucose (n = 4–10). A positive control for cleaved (Cl.) caspase-3 supplied by the manufacturer (lysates from apoptotic T-cells) was included in the final lane. #P < 0.05 vs. 5 mmol/l glucose control; *P < 0.05 vs. 25 mmol/l glucose control.
, 100 μmol/l ryanodine + 1 μmol/l xestaspongin C; •, 1 μmol/l thapsigargin. B: MIN6 cells were cultured as indicated and probed for markers of ER stress and apoptosis, as in Fig. 2. CHOP expression was examined and quantified at both low and high glucose (n = 4–10). C: Cleaved caspase-3 expression examined and quantified at both low and high glucose (n = 4–10). A positive control for cleaved (Cl.) caspase-3 supplied by the manufacturer (lysates from apoptotic T-cells) was included in the final lane. #P < 0.05 vs. 5 mmol/l glucose control; *P < 0.05 vs. 25 mmol/l glucose control.
FIG. 4.
ER Ca2+ channel blockers can reduce ER stress and apoptosis induced by thapsigargin (Tg). A and B: Cell death induced by 1 μmol/l thapsigargin was significantly attenuated by 30 μmol/l of the RyR1 inhibitor dantrolene (n = 4). A: ○, control; ▴, 30 μmol/l dantrolene; •, 1 μmol/l thapsigargin;  , 1 μmol/l thapsigargin + 30 μmol/l dantrolene. A trend toward protection from thapsigargin-induced death was also observed in response to treatment with 100 μmol/l ryanodine alone (n = 4) or in combination with 1 μmol/l xestospongin C (n = 6). C and D: Caspase-3 activation following an 8-h treatment with thapsigargin was reduced by dantrolene (n = 3). E: Caspase-3 cleavage in MIN6 cells cultured at 5 mmol/l glucose for 24 h with 1 μmol/l thapsigargin in the presence or absence of both 100 μmol/l ryanodine and 1 μmol/l xestospongin C (n = 6). F and G: Quantified Western blots of cleaved caspase-3 and CHOP levels in MIN6 cells treated for 8 h in 5 mmol/l glucose as indicated (n = 3). #P < 0.05 vs. control; *P < 0.05 vs. thapsigargin alone.
, 1 μmol/l thapsigargin + 30 μmol/l dantrolene. A trend toward protection from thapsigargin-induced death was also observed in response to treatment with 100 μmol/l ryanodine alone (n = 4) or in combination with 1 μmol/l xestospongin C (n = 6). C and D: Caspase-3 activation following an 8-h treatment with thapsigargin was reduced by dantrolene (n = 3). E: Caspase-3 cleavage in MIN6 cells cultured at 5 mmol/l glucose for 24 h with 1 μmol/l thapsigargin in the presence or absence of both 100 μmol/l ryanodine and 1 μmol/l xestospongin C (n = 6). F and G: Quantified Western blots of cleaved caspase-3 and CHOP levels in MIN6 cells treated for 8 h in 5 mmol/l glucose as indicated (n = 3). #P < 0.05 vs. control; *P < 0.05 vs. thapsigargin alone.
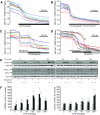
FIG. 5.
IP3 and ryanodine receptor activation augments β-cell death and ER stress. A_–_C: Cholinergic activation of IP3R by 100 μmol/l carbachol (Cch; n = 6) or activation of RyR with 1 nmol/l ryanodine (n = 3) increased cell death in response to SERCA inhibition with 1 μmol/l thapsigargin (Tg). A: ○, control; ▴, 100 μmol/l Cch; •, 1 μmol/l thapsigargin;  , 1 μmol/l thapsigargin + 100 μmol/l Cch. B: ○, control; •, 1 μmol/l thapsigargin;
, 1 μmol/l thapsigargin + 100 μmol/l Cch. B: ○, control; •, 1 μmol/l thapsigargin;  , 1 μmol/l thapsigargin + 1 nmol/l ryanodine. D and E: MIN6 cells cultured for 8 h with a combination of 100 μmol/l carbachol (Cch) and 1 μmol/l thapsigargin showed increased ER stress (CHOP expression), compared with either treatment alone (n = 6). #P < 0.05 vs. control; *P < 0.05 vs. thapsigargin alone.
, 1 μmol/l thapsigargin + 1 nmol/l ryanodine. D and E: MIN6 cells cultured for 8 h with a combination of 100 μmol/l carbachol (Cch) and 1 μmol/l thapsigargin showed increased ER stress (CHOP expression), compared with either treatment alone (n = 6). #P < 0.05 vs. control; *P < 0.05 vs. thapsigargin alone.
FIG. 6.
Effects of IP3 receptor activation on the ER Ca2+ depletion and unfolded protein response activation evoked by SERCA inhibition. A and B: D1ER cameleon measurements of the luminal ER Ca2+ release induced by 1 μmol/l thapsigargin, with or without simultaneous addition of 100 μmol/l carbachol (Cch) (n = 10 and 14 cells, respectively). C: Effects of 1 μmol/l thapsigargin administered during exposure to 100 μmol/l Cch (n = 8 cells). D: Effects of 100 μmol/l Cch administered in the presence of 1 μmol/l thapsigargin (n = 9 cells). Note the acceleration of ER Ca2+ depletion in cells that had not yet reached a stable depleted state (arrow). E: Phosphorylation of PERK and eIF2α in 25 mmol/l glucose-cultured MIN6 cells was examined at the time points indicated. F: Quantification of Western blots for PERK and eIF2α phosphorylation. Similar results from experiments conducted in 5 and 25 mmol/l glucose were pooled (n = 6, #P < 0.05 vs. control). □, control;  , 100 μmol/l Cch; ▪, 1 μmol/l thapsigargin;
, 100 μmol/l Cch; ▪, 1 μmol/l thapsigargin;  , thapsigargin + Cch. (Please see
, thapsigargin + Cch. (Please see
http://dx.doi.org/10.2337/db07-1762
for a high-quality digital representation of this figure).
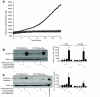
FIG. 7.
Time-dependent effects of ER Ca2+ depletion on mitochondria. Mitochondrial membrane potential was monitored by flow cytometry analysis of TMRE-stained MIN6 cells. A: Representative histograms illustrating that SERCA inhibition rapidly induces mitochondrial hyperpolarization followed later (>24 h) by the collapse of mitochondrial polarization. For quantification, depolarized, intermediate, and hyperpolarized cell populations were defined as indicated in the first panel. CCCP-treated cells are shown as a control for mitochondrial depolarization. B: Quantification of the time and treatment dependence of the fraction of cells in the depolarized and hyperpolarized mitochondrial states. (n = 12 for CCCP, n = 3–4 at each time point for all other treatments; #P < 0.05 vs. control at the same time point, *P < 0.05 vs. thapsigargin alone at the same time point.) ▪, depolarized; □, hyperpolarized. C: Western blots of cleaved caspase-9 levels 24 or 48 h following treatments as indicated. Cleaved caspase-9 normalized to actin (in arbitrary units): 24 h, control 0.42 ± 0.17 vs. 1 μmol/l thapsigargin, 1.01 ± 0.17, P < 0.05, n = 4; 48 h, control 0.62 ± 0.13 vs. thapsigargin, 2.20 ± 0.48, P < 0.05, n = 4. D: Example of mitochondrial Ca2+ responses in a MIN6 cell following mobilization of ER Ca2+ by 100 μmol/l carbachol or 1 μmol/l thapsigargin.
FIG. 8.
Effects of SERCA, RyR, and IP3R inhibition on death of primary mouse islet cells. A: Representative real-time measurement of the propidium iodide incorporation in primary mouse islet cells exposed to 1 or 10 μmol/l thapsigargin. ○, control; ▴, 1 μmol/l thapsigargin; •, 10 μmol/l thapsigargin. B: Summary of the islet cell death induced by 0.1 μmol/l (n = 3), 1 μmol/l (n = 5), and 10 μmol/l (n = 3) thapsigargin. C: Summary of the effects of 100 μmol/l carbachol, 1 μmol/l xestospongin C, and 100 μmol/l ryanodine on the mouse islet cell death induced by 24 h exposure to 1 μmol/l thapsigargin (n = 3). #P < 0.05 vs. control; *P < 0.05 vs. thapsigargin alone.
Similar articles
- Endoplasmic reticulum stress alters ryanodine receptor function in the murine pancreatic β cell.
Yamamoto WR, Bone RN, Sohn P, Syed F, Reissaus CA, Mosley AL, Wijeratne AB, True JD, Tong X, Kono T, Evans-Molina C. Yamamoto WR, et al. J Biol Chem. 2019 Jan 4;294(1):168-181. doi: 10.1074/jbc.RA118.005683. Epub 2018 Nov 12. J Biol Chem. 2019. PMID: 30420428 Free PMC article. - Hydroxylated xestospongins block inositol-1,4,5-trisphosphate-induced Ca2+ release and sensitize Ca2+-induced Ca2+ release mediated by ryanodine receptors.
Ta TA, Feng W, Molinski TF, Pessah IN. Ta TA, et al. Mol Pharmacol. 2006 Feb;69(2):532-8. doi: 10.1124/mol.105.019125. Epub 2005 Oct 25. Mol Pharmacol. 2006. PMID: 16249374 - Sarco-Endoplasmic Reticulum Calcium Release Model Based on Changes in the Luminal Calcium Content.
Guerrero-Hernández A, Sánchez-Vázquez VH, Martínez-Martínez E, Sandoval-Vázquez L, Perez-Rosas NC, Lopez-Farias R, Dagnino-Acosta A. Guerrero-Hernández A, et al. Adv Exp Med Biol. 2020;1131:337-370. doi: 10.1007/978-3-030-12457-1_14. Adv Exp Med Biol. 2020. PMID: 31646517 Review. - Alterations of the Endoplasmic Reticulum (ER) Calcium Signaling Molecular Components in Alzheimer's Disease.
Chami M, Checler F. Chami M, et al. Cells. 2020 Dec 1;9(12):2577. doi: 10.3390/cells9122577. Cells. 2020. PMID: 33271984 Free PMC article. Review.
Cited by
- Endoplasmic reticulum stress alters ryanodine receptor function in the murine pancreatic β cell.
Yamamoto WR, Bone RN, Sohn P, Syed F, Reissaus CA, Mosley AL, Wijeratne AB, True JD, Tong X, Kono T, Evans-Molina C. Yamamoto WR, et al. J Biol Chem. 2019 Jan 4;294(1):168-181. doi: 10.1074/jbc.RA118.005683. Epub 2018 Nov 12. J Biol Chem. 2019. PMID: 30420428 Free PMC article. - Apoptosis and autophagy: decoding calcium signals that mediate life or death.
Harr MW, Distelhorst CW. Harr MW, et al. Cold Spring Harb Perspect Biol. 2010 Oct;2(10):a005579. doi: 10.1101/cshperspect.a005579. Epub 2010 Sep 8. Cold Spring Harb Perspect Biol. 2010. PMID: 20826549 Free PMC article. Review. - Endoplasmic Reticulum (ER) Stress and Its Role in Pancreatic β-Cell Dysfunction and Senescence in Type 2 Diabetes.
Lee JH, Lee J. Lee JH, et al. Int J Mol Sci. 2022 Apr 27;23(9):4843. doi: 10.3390/ijms23094843. Int J Mol Sci. 2022. PMID: 35563231 Free PMC article. Review. - Osthole Suppresses Cell Growth of Prostate Cancer by Disrupting Redox Homeostasis, Mitochondrial Function, and Regulation of tiRNAHisGTG.
Song J, Ham J, Song G, Lim W. Song J, et al. Antioxidants (Basel). 2024 May 30;13(6):669. doi: 10.3390/antiox13060669. Antioxidants (Basel). 2024. PMID: 38929108 Free PMC article. - Npas4 is a novel activity-regulated cytoprotective factor in pancreatic β-cells.
Sabatini PV, Krentz NA, Zarrouki B, Westwell-Roper CY, Nian C, Uy RA, Shapiro AM, Poitout V, Lynn FC. Sabatini PV, et al. Diabetes. 2013 Aug;62(8):2808-20. doi: 10.2337/db12-1527. Epub 2013 May 8. Diabetes. 2013. PMID: 23656887 Free PMC article.
References
- Harding HP, Ron D: Endoplasmic reticulum stress and the development of diabetes: a review. Diabetes 51 (Suppl. 3): S455–S461, 2002 - PubMed
- Huang CJ, Lin CY, Haataja L, Gurlo T, Butler AE, Rizza RA, Butler PC: High expression rates of human islet amyloid polypeptide induce endoplasmic reticulum stress–mediated β-cell apoptosis, a characteristic of humans with type 2 but not type 1 diabetes. Diabetes 56: 2016–2027, 2007 - PubMed
- Cnop M, Welsh N, Jonas JC, Jorns A, Lenzen S, Eizirik DL: Mechanisms of pancreatic β-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes 54 (Suppl. 2): S97–S107, 2005 - PubMed
- Yamada T, Ishihara H, Tamura A, Takahashi R, Yamaguchi S, Takei D, Tokita A, Satake C, Tashiro F, Katagiri H, Aburatani H, Miyazaki J, Oka Y: WFS1-deficiency increases endoplasmic reticulum stress, impairs cell cycle progression and triggers the apoptotic pathway specifically in pancreatic β-cells. Hum Mol Genet 15: 1600–1609, 2006 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous