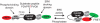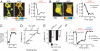A genetically encoded fluorescent sensor of ERK activity - PubMed (original) (raw)
A genetically encoded fluorescent sensor of ERK activity
Christopher D Harvey et al. Proc Natl Acad Sci U S A. 2008.
Abstract
The activity of the ERK has complex spatial and temporal dynamics that are important for the specificity of downstream effects. However, current biochemical techniques do not allow for the measurement of ERK signaling with fine spatiotemporal resolution. We developed a genetically encoded, FRET-based sensor of ERK activity (the extracellular signal-regulated kinase activity reporter, EKAR), optimized for signal-to-noise ratio and fluorescence lifetime imaging. EKAR selectively and reversibly reported ERK activation in HEK293 cells after epidermal growth factor stimulation. EKAR signals were correlated with ERK phosphorylation, required ERK activity, and did not report the activities of JNK or p38. EKAR reported ERK activation in the dendrites and nucleus of hippocampal pyramidal neurons in brain slices after theta-burst stimuli or trains of back-propagating action potentials. EKAR therefore permits the measurement of spatiotemporal ERK signaling dynamics in living cells, including in neuronal compartments in intact tissues.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Schematic of EKAR. ERK phosphorylation of EKAR triggers a conformational change and an increase in FRET between EGFP and mRFP1.
Fig. 2.
Function of EKAR. (A) (Left) Fluorescence lifetime images of HEK293 cells transfected with EKARcyto before (−5 min) and after (12 min) addition of EGF (100 ng/ml). (Right) Time course of the EGF-induced ERK activation. (B) (Left) Fluorescence lifetime images of HEK293 cells transfected with EKARcyto and EKARnuclear before (−4 min) and after (15 min) addition of EGF. EKAR concentration was higher in the nucleus. (Right) Time course of ERK activation in the nucleus and cytoplasm. (C) EGF-induced ERK activation, measured as the ratio of acceptor-to-donor fluorescence, in HEK293 cells expressing a CFP-YFP version of EKARcyto. Each region-of-interest (ROI) contained 2–6 cells. Data are mean ± SEM for 5 ROIs from 5 dishes. (D) EGF-induced lifetime changes in HEK293 cells expressing EKARcyto variants containing docking sites with different affinities for ERK. _K_m values are from ref. . Data for each docking site are mean ± SEM for ≥5 ROIs from 2 dishes. (E) Lifetime changes in HEK293 cells expressing central linker variants of EKARcyto and an EGFP-mRFP1 version of AKAR2 after EGF stimuli or application of the adenylate cyclase activator forskolin (25 μM) and the phosphodiesterase inhibitor IBMX (100 μM), respectively. The sequence of the short glycine-rich linker is GNNGGNGGS (7) for EKAR and SAGKPGSGEGSTKG for AKAR2 (21). Data are mean ± SEM for ≥7 ROIs from ≥2 dishes for each condition. (F) EGF-induced lifetime changes for EKARcyto and EKARcyto mutated (Thr-to-Ala) at the phosphorylation site in the substrate peptide. For the wild type sensor, 10 μM U0126 was added 65 min after EGF application. Data are mean ± SEM for ≥9 ROIs from ≥2 dishes for each condition.
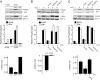
Fig. 3.
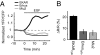
Analysis of the relationship between ERK phosphorylation, EKAR phosphorylation, and EKAR signals. (A) Correlation between ERK phosphorylation, EKAR phosphorylation, and EKAR signals in COS7 cells. (Top and Middle) Cells were transfected with EKARcyto or EKARcyto (Thr-to-Ala). ERK activity was modulated by cotransfection with a constitutively active MEK (ΔMEK1) or a MAPK phosphatase (MKP1). An example Western blot is shown. Relative phosphorylation is the ratio of phosphorylated protein to total protein. Data are mean ± SEM from 3 experiments. (Bottom) Fluorescence lifetimes in cells expressing EKARcyto in control, ΔMEK1, and MKP1 conditions. Data for each condition are mean ± SEM for >20 cells from 2 dishes. (B) PMA-induced ERK phosphorylation, EKAR phosphorylation, and EKAR signals in COS7 cells transfected with EKARcyto. Cells were stimulated with PMA (1 μM; 10 min). MAPK pathways were blocked by preincubation (30 min) with ERK (10 μM U0126), JNK (10 μM SP600125), and p38 (10 μM PD169316) pathway inhibitors. (Top and Middle) An example Western blot is shown. Data are mean ± SEM from 3 experiments. (Bottom) Changes in lifetime after PMA application with and without U0126 (10 μM). Data for each condition are mean ± SEM for 10 cells from 2 dishes. (C) UV irradiation-induced ERK phosphorylation, EKAR phosphorylation, and EKAR signals in COS7 cells transfected with EKARcyto. Cells were irradiated with 60 J/m2 UV-C and then incubated for 1 h. Drugs are the same as in B. (Top and Middle) An example Western blot is shown. Data are mean ± SEM from 3 experiments. (Bottom) Fluorescence lifetimes in cells expressing EKARcyto after UV-C treatment with and without U0126 (10 μM). Data for each condition are mean ± SEM for >20 cells from 2 dishes.
Fig. 4.
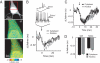
EKAR function in hippocampal neurons. (A) Fluorescence lifetime images of dendrites from a pyramidal neuron in a cultured hippocampal brain slice before and after trains of back-propagating action potentials (40 APs at 83 Hz, repeated 8 times at 0.2 Hz). (B) Time course of ERK activity. Each ROI contained a ≈20-μm stretch of apical dendrite <70 μm from the soma. In the example image, the entire field of view was used as the ROI. Data are mean ± SEM for 7 cells.
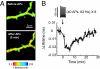
Fig. 5.
ERK activation in the cytoplasm and nucleus after theta-burst stimuli. (A) (Top) GFP fluorescence image of a hippocampal pyramidal neuron expressing EKARcyto and EKARnuclear in a cultured brain slice. (Middle and Bottom) Fluorescence lifetime images of the same cell (boxed area). Images are from before (−5 min) and after (8 min) theta-burst stimuli. (B) Changes in lifetime in the cytoplasm and nucleus for the example shown in A. At time = 0, theta-burst stimuli (5 synaptic stimuli at 100 Hz, repeated 10 times at 5 Hz) were delivered 3 times with 10-second intervals. Example perforated patch recordings at the soma are shown. ROIs for the cytoplasm and nucleus were distinguished based on fluorescence intensity. (C) Time course of ERK activation in the nucleus and cytoplasm after theta-burst stimuli. Data are from 7 cells. (D) Lifetime changes following theta-burst stimuli in the nucleus and cytoplasm in the presence or absence of the L-type VGCC blocker nimodipine (20 μM). Lifetime changes were the average of 3 time points around the maximum change within 15 min of the stimulus. Data are from 7 and 6 cells for control and nimodipine conditions, respectively.
Fig. 6.
Comparison of ERK activity reporters. (A) EGF-induced changes in the acceptor-to-donor fluorescence ratio in HEK293 cells expressing CFP-YFP versions of EKARcyto, Erkuscyto, or Miu2. Example traces from individual experiments are shown. (B) Ratio changes for CFP-YFP versions of EKARcyto, Erkuscyto, and Miu2 in HEK293 cells after EGF application. The ratio, R, was measured as the YFP/CFP ratio for EKARcyto and the CFP/YFP ratio for Erkuscyto and Miu2. Data are mean ± SEM for ≥5 ROIs from 5 dishes.
Fig. 7.
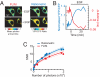
Signal-to-noise ratios of FLIM and intensity-based FRET measurements. (A) FLIM and YFP/CFP ratio images of HEK293 cells transfected with a CFP-YFP version of EKARcyto before and after EGF (100 ng/ml) application. The FLIM image is color-coded based on mean photon arrival times (see
SI Text
). (B) Lifetime (red) and ratio (blue) changes for the example shown in A. (C) SNRs of FLIM and ratiometric measurements at various numbers of donor photons (N). Each data point represents a single experiment; FLIM and intensity images were interleaved. The data were fitted by using the relationship SNR ∼ (N)1/2. Light blue and light red lines indicate the 95% confidence intervals.
Similar articles
- Pattern-dependent role of NMDA receptors in action potential generation: consequences on extracellular signal-regulated kinase activation.
Zhao M, Adams JP, Dudek SM. Zhao M, et al. J Neurosci. 2005 Jul 27;25(30):7032-9. doi: 10.1523/JNEUROSCI.1579-05.2005. J Neurosci. 2005. PMID: 16049179 Free PMC article. - Measuring ERK Activity Dynamics in Single Living Cells Using FRET Biosensors.
Blum Y, Fritz RD, Ryu H, Pertz O. Blum Y, et al. Methods Mol Biol. 2017;1487:203-221. doi: 10.1007/978-1-4939-6424-6_15. Methods Mol Biol. 2017. PMID: 27924569 - Automated FRET quantification shows distinct subcellular ERK activation kinetics in response to graded EGFR signaling in Drosophila.
Ogura Y, Sami MM, Wada H, Hayashi S. Ogura Y, et al. Genes Cells. 2019 Apr;24(4):297-306. doi: 10.1111/gtc.12679. Genes Cells. 2019. PMID: 30851218 - Shedding light on developmental ERK signaling with genetically encoded biosensors.
Nakamura A, Goto Y, Kondo Y, Aoki K. Nakamura A, et al. Development. 2021 Sep 15;148(18):dev199767. doi: 10.1242/dev.199767. Epub 2021 Aug 2. Development. 2021. PMID: 34338283 Review. - ERK Activity Imaging During Migration of Living Cells In Vitro and In Vivo.
Hirata E, Kiyokawa E. Hirata E, et al. Int J Mol Sci. 2019 Feb 5;20(3):679. doi: 10.3390/ijms20030679. Int J Mol Sci. 2019. PMID: 30764494 Free PMC article. Review.
Cited by
- Studying signal transduction in single dendritic spines.
Yasuda R. Yasuda R. Cold Spring Harb Perspect Biol. 2012 Oct 1;4(10):a005611. doi: 10.1101/cshperspect.a005611. Cold Spring Harb Perspect Biol. 2012. PMID: 22843821 Free PMC article. Review. - Emerging roles and mechanisms of ERK pathway mechanosensing.
Crozet F, Levayer R. Crozet F, et al. Cell Mol Life Sci. 2023 Nov 10;80(12):355. doi: 10.1007/s00018-023-05007-z. Cell Mol Life Sci. 2023. PMID: 37947896 Free PMC article. Review. - Biasing the prostaglandin F2α receptor responses toward EGFR-dependent transactivation of MAPK.
Goupil E, Wisehart V, Khoury E, Zimmerman B, Jaffal S, Hébert TE, Laporte SA. Goupil E, et al. Mol Endocrinol. 2012 Jul;26(7):1189-202. doi: 10.1210/me.2011-1245. Epub 2012 May 25. Mol Endocrinol. 2012. PMID: 22638073 Free PMC article. - Bidirectional in vivo structural dendritic spine plasticity revealed by two-photon glutamate uncaging in the mouse neocortex.
Noguchi J, Nagaoka A, Hayama T, Ucar H, Yagishita S, Takahashi N, Kasai H. Noguchi J, et al. Sci Rep. 2019 Sep 26;9(1):13922. doi: 10.1038/s41598-019-50445-0. Sci Rep. 2019. PMID: 31558759 Free PMC article. - Genetically encoded fluorescent sensors for imaging neuronal dynamics in vivo.
Day-Cooney J, Dalangin R, Zhong H, Mao T. Day-Cooney J, et al. J Neurochem. 2023 Feb;164(3):284-308. doi: 10.1111/jnc.15608. Epub 2022 Apr 9. J Neurochem. 2023. PMID: 35285522 Free PMC article. Review.
References
- Thomas GM, Huganir RL. MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci. 2004;5:173–183. - PubMed
- Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. - PubMed
- Yasuda R. Imaging spatiotemporal dynamics of neuronal signaling using fluorescence resonance energy transfer and fluorescence lifetime imaging microscopy. Curr Opin Neurobiol. 2006;16:551–561. - PubMed
- Miyawaki A. Visualization of the spatial and temporal dynamics of intracellular signaling. Dev Cell. 2003;4:295–305. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous