Real-time assessment of inflammation and treatment response in a mouse model of allergic airway inflammation - PubMed (original) (raw)
. 2008 Dec;118(12):4058-66.
doi: 10.1172/JCI36335. Epub 2008 Nov 6.
Filip K Swirski, Peter Waterman, Hushan Yuan, Jose Luiz Figueiredo, Andita P Newton, Rabi Upadhyay, Claudio Vinegoni, Rainer Kohler, Joseph Blois, Adam Smith, Matthias Nahrendorf, Lee Josephson, Ralph Weissleder, Mikael J Pittet
Affiliations
- PMID: 19033674
- PMCID: PMC2579705
- DOI: 10.1172/JCI36335
Real-time assessment of inflammation and treatment response in a mouse model of allergic airway inflammation
Virna Cortez-Retamozo et al. J Clin Invest. 2008 Dec.
Abstract
Eosinophils are multifunctional leukocytes that degrade and remodel tissue extracellular matrix through production of proteolytic enzymes, release of proinflammatory factors to initiate and propagate inflammatory responses, and direct activation of mucus secretion and smooth muscle cell constriction. Thus, eosinophils are central effector cells during allergic airway inflammation and an important clinical therapeutic target. Here we describe the use of an injectable MMP-targeted optical sensor that specifically and quantitatively resolves eosinophil activity in the lungs of mice with experimental allergic airway inflammation. Through the use of real-time molecular imaging methods, we report the visualization of eosinophil responses in vivo and at different scales. Eosinophil responses were seen at single-cell resolution in conducting airways using near-infrared fluorescence fiberoptic bronchoscopy, in lung parenchyma using intravital microscopy, and in the whole body using fluorescence-mediated molecular tomography. Using these real-time imaging methods, we confirmed the immunosuppressive effects of the glucocorticoid drug dexamethasone in the mouse model of allergic airway inflammation and identified a viridin-derived prodrug that potently inhibited the accumulation and enzyme activity of eosinophils in the lungs. The combination of sensitive enzyme-targeted sensors with noninvasive molecular imaging approaches permitted evaluation of airway inflammation severity and was used as a model to rapidly screen for new drug effects. Both fluorescence-mediated tomography and fiberoptic bronchoscopy techniques have the potential to be translated into the clinic.
Figures
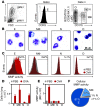
Figure 1. Eosinophils in inflamed lungs display potent MMP activity.
E, eosinophils; Mf, monocytes/macrophages; N, neutrophils, O, other cells. (A) Identification of cell populations in single-cell suspensions of digested lungs from OVA-treated and control PBS-treated mice. FSC, forward scatter; SSC, side scatter. (B) H&E staining of flow-sorted cells obtained from digested lung tissue and identified based on expression of specific cell surface markers. Scale bar: 25 μm. (C) Flow cytometric analysis revealed increased MMP activity in eosinophils in inflamed lungs. Numbers denote percent MMP-positive cells in inflamed versus control lung. (D) Total cell counts in lungs. (E) MMP mean fluorescence intensity (MFI) in each cell type in lung, as identified by flow cytometry. (F) Contribution of cell types to MMP activity in inflamed lungs based on results in D and E. Data are representative of at least 3 independent experiments, with n = 3–5 per experiment and per group. Data are mean ± SEM.
Figure 2. In vivo detection of eosinophil-mediated MMP activity correlates with disease severity.
Sens, sensitization (×1, once; ×2, twice); Chall, challenge with OVA. (A) IVM showed single-cell MMP activity in inflamed versus control lungs. (B) Virtual 3-dimensional rendering of MMP activity in inflamed lungs of 2 different mice and acquired by FMT-CT. MMP activity localized centrally to the lower trachea and major bronchi in mouse 1, and to the right and left lobes of the lung in mouse 2. (C and D) FMT measurement of MMP activity in whole lungs of mice with no (unchallenged; blue), moderate (sensitized once with OVA/alum; orange), or potent (sensitized twice with OVA/alum; red) airway inflammation. Mice 1 and 2 from B are shown. (E) MMP activity in vivo positively correlated with the number of eosinophils in BAL fluids. (F and G) FRI of excised lungs confirmed the FMT results. At least 3 independent experiments were performed, with n = 3–5 per experiment and per group. Data are mean ± SEM. *P < 0.05, **P < 0.01 versus once-sensitized group. Scale bars: 50 μm (A); 1 cm (B).
Figure 3. Decreased eosinophil-mediated MMP activity in MMP-12–deficient mice.
(A) MMP-12–deficient (KO, green) and wild-type mice (red) recruited eosinophils to the inflamed lung. Unchallenged mice (blue) served as controls. (B) MMP-12–deficient mice showed decreased MMP activity in lung eosinophils, as identified by flow cytometry. (C and D) FMT imaging revealed decreased MMP activity in inflamed lungs of MMP-12–deficient mice. At least 2 independent experiments were performed, with n = 3 per experiment and per group. Data are mean ± SEM. *P < 0.05 versus OVA-challenged WT.
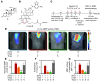
Figure 4. In vivo FMT for detection of treatment response.
(A) Structure of dexamethasone (D). (B) Structures of the self-activating viridin prodrug (S; R = CH3) and control nonactivating compound (N; R = H). (C) Protocol of procedures and treatment regimen. (D and E) FMT informs on treatment efficacy. Dexamethasone (orange) and self-activating viridin prodrug (green) suppressed eosinophil-associated MMP activity in lung compared with control mice treated with vehicle dextran alone (V; red) or control nonactivating compound (gray). Unchallenged mice (blue) served as controls. (F) Ex vivo analysis of digested lungs. Administration of dexamethasone or self-activating viridin prodrug decreased the number of lung eosinophils. (G) Flow cytometry analysis of digested lungs revealed that administration of self-activating viridin prodrug, and, to a lesser extent, dexamethasone, decreased MMP activity of eosinophils on a per-cell basis. ND, not done. At least 2 independent experiments were performed, with n = 3–5 per experiment and per group. Data are mean ± SEM. *P < 0.05, **P < 0.01 versus OVA-challenged, vehicle dextran–treated control.
Figure 5. In vivo NIRF bronchoscopy for detection of treatment response.
(A) Schematic representation of the airways and features of imaging modalities. FMT resolves signals coming from the entire organ, whereas NIRF fiberoptic bronchoscopy resolves signals coming mostly from the conducting airways. Imaging resolutions are 1 mm and 15 μm for FMT and bronchoscopy, respectively. (B and C) Microcatheter-based NIRF bronchoscopy quantitatively informed on MMP activity within bronchi of mice sensitized and challenged with OVA. (B) Third-order branches are shown. Scale bar: 50 μm. (C) The signal was dramatically reduced in mice administered dexamethasone (orange) or the self-activating viridin prodrug (green) compared with mice administered vehicle dextran only (red). Unchallenged mice (blue) served as controls. (D) In vivo MMP activity detected by NIRF bronchoscopy positively correlated with the number of eosinophils in BAL fluids (_r_2= 0.79). At least 2 independent experiments were performed, with n = 3–5 per experiment and per group. Data are mean ± SEM. *P < 0.05 versus OVA-challenged, vehicle dextran–treated control.
Similar articles
- Antiallergic and anti-inflammatory effects of a novel I kappaB kinase beta inhibitor, IMD-0354, in a mouse model of allergic inflammation.
Sugita A, Ogawa H, Azuma M, Muto S, Honjo A, Yanagawa H, Nishioka Y, Tani K, Itai A, Sone S. Sugita A, et al. Int Arch Allergy Immunol. 2009;148(3):186-98. doi: 10.1159/000161579. Epub 2008 Oct 10. Int Arch Allergy Immunol. 2009. PMID: 18849610 - A novel murine model of allergic inflammation to study the effect of dexamethasone on eosinophil recruitment.
Das AM, Flower RJ, Hellewell PG, Teixeira MM, Perretti M. Das AM, et al. Br J Pharmacol. 1997 May;121(1):97-104. doi: 10.1038/sj.bjp.0701122. Br J Pharmacol. 1997. PMID: 9146893 Free PMC article. - Effect of tecastemizole on pulmonary and cutaneous allergic inflammatory responses.
Lever R, Hefni A, Moffatt JD, Paul W, Page CP. Lever R, et al. Clin Exp Allergy. 2007 Jun;37(6):909-17. doi: 10.1111/j.1365-2222.2007.02730.x. Clin Exp Allergy. 2007. PMID: 17517105 - Eosinophilopoiesis at the cross-roads of research on development, immunity and drug discovery.
Elsas PX, Elsas MI. Elsas PX, et al. Curr Med Chem. 2007;14(18):1925-39. doi: 10.2174/092986707781368487. Curr Med Chem. 2007. PMID: 17691935 Review. - Role of IgE in the development of allergic airway inflammation and airway hyperresponsiveness--a murine model.
Hamelmann E, Tadeda K, Oshiba A, Gelfand EW. Hamelmann E, et al. Allergy. 1999 Apr;54(4):297-305. doi: 10.1034/j.1398-9995.1999.00085.x. Allergy. 1999. PMID: 10371087 Review.
Cited by
- In vivo imaging of tracheal epithelial cells in mice during airway regeneration.
Kim JK, Vinarsky V, Wain J, Zhao R, Jung K, Choi J, Lam A, Pardo-Saganta A, Breton S, Rajagopal J, Yun SH. Kim JK, et al. Am J Respir Cell Mol Biol. 2012 Dec;47(6):864-8. doi: 10.1165/rcmb.2012-0164OC. Epub 2012 Sep 13. Am J Respir Cell Mol Biol. 2012. PMID: 22984086 Free PMC article. - Pre-clinical whole-body fluorescence imaging: Review of instruments, methods and applications.
Leblond F, Davis SC, Valdés PA, Pogue BW. Leblond F, et al. J Photochem Photobiol B. 2010 Jan 21;98(1):77-94. doi: 10.1016/j.jphotobiol.2009.11.007. Epub 2009 Nov 26. J Photochem Photobiol B. 2010. PMID: 20031443 Free PMC article. Review. - Heterogeneous in vivo behavior of monocyte subsets in atherosclerosis.
Swirski FK, Weissleder R, Pittet MJ. Swirski FK, et al. Arterioscler Thromb Vasc Biol. 2009 Oct;29(10):1424-32. doi: 10.1161/ATVBAHA.108.180521. Epub 2009 Apr 16. Arterioscler Thromb Vasc Biol. 2009. PMID: 19372462 Free PMC article. Review. - Non-invasive optical imaging of eosinophilia during the course of an experimental allergic airways disease model and in response to therapy.
Markus MA, Dullin C, Mitkovski M, Prieschl-Grassauer E, Epstein MM, Alves F. Markus MA, et al. PLoS One. 2014 Feb 25;9(2):e90017. doi: 10.1371/journal.pone.0090017. eCollection 2014. PLoS One. 2014. PMID: 24587190 Free PMC article. - Functionalized synchrotron in-line phase-contrast computed tomography: a novel approach for simultaneous quantification of structural alterations and localization of barium-labelled alveolar macrophages within mouse lung samples.
Dullin C, dal Monego S, Larsson E, Mohammadi S, Krenkel M, Garrovo C, Biffi S, Lorenzon A, Markus A, Napp J, Salditt T, Accardo A, Alves F, Tromba G. Dullin C, et al. J Synchrotron Radiat. 2015 Jan;22(1):143-55. doi: 10.1107/S1600577514021730. Epub 2015 Jan 1. J Synchrotron Radiat. 2015. PMID: 25537601 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources