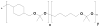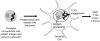The stimulation of CD8+ T cells by dendritic cells pulsed with polyketal microparticles containing ion-paired protein antigen and poly(inosinic acid)-poly(cytidylic acid) - PubMed (original) (raw)
The stimulation of CD8+ T cells by dendritic cells pulsed with polyketal microparticles containing ion-paired protein antigen and poly(inosinic acid)-poly(cytidylic acid)
Michael J Heffernan et al. Biomaterials. 2009 Feb.
Abstract
New adjuvants and delivery strategies are needed to optimize the ability of protein-based vaccines to elicit CD8(+) T cell responses. We have developed a model vaccine formulation containing ovalbumin (OVA) and the double-stranded RNA analog poly(inosinic acid)-poly(cytidylic acid) (poly(I:C)), a TLR3 agonist. OVA and poly(I:C) were each ion-paired to cetyltrimethylammonium bromide (CTAB) to produce hydrophobic complexes, which were co-encapsulated in pH-sensitive polyketal (PK3) microparticles (1-3 microm) using a single emulsion method. Loading levels ranged from 13.6 to 18.8 microg/mg OVA and 4.8 to 10.3 microg/mg poly(I:C). Murine splenic dendritic cells (DCs) pulsed with PK3-OVA-poly(I:C) microparticles, at antigen doses of 0.01 and 0.1 microg/mL, induced a higher percentage of IFNgamma-producing CD8(+) T cells than DCs treated with PK3-OVA particles or soluble OVA/poly(I:C). A higher antigen dose (1 microg/mL) was less effective, which can be attributed to CTAB toxicity. At the lowest antigen dose (0.01 microg/mL), PK3-OVA-poly(I:C) microparticles also enhanced TNF-alpha and IL-2 production in CD8(+) T cells. These data demonstrate the potential of polyketal microparticles in formulating effective CD8(+) T cell-inducing vaccines comprising protein antigens and dsRNA adjuvants.
Figures
Fig. 1
Structure of co-polyketal PK3, which is synthesized from 1,4-cyclohexanedimethanol, 1,5-pentanediol, and 2,2-dimethoxypropane via the acetal exchange polymerization [32,34].
Fig. 2
Biodegradable polyketal microparticles containing protein antigen and poly(I:C) adjuvant are phagocytosed by dendritic cells (DCs). pH-Sensitive polyketal degrades and releases protein antigen and dsRNA analog poly(I:C) in phagolysosome. Poly(I:C) engages TLR3, thereby activating DC and cross-priming CD8+ cytotoxic T lymphocytes.
Fig. 3
Hydrophobic ion-pairing procedure. (A) Poly(I:C) solution or (B) OVA solution at pH 11 is paired with CTAB in equimolar ratio of opposite charges to form a hydrophobic complex.
Fig. 4
Scanning electron microscope image of polyketal microparticles containing ion-paired OVA and poly(I:C).
Fig. 5
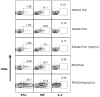
Cell viability of RAW264.7 macrophages treated for 5 h with (A) PK3 micro-particles containing OVA and/or poly(I:C) or empty microparticles or (B) soluble OVA, poly(I:C), or CTAB.
Fig. 6
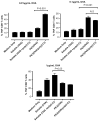
Representative flow cytometry plots showing percentage of IFNγ-, TNFα- and IL-2-producing CD8+ T cells stimulated by DCs pulsed with PK3-encapsulated OVA and poly(I:C) (0.01 μg/mL antigen dose).
Fig. 7
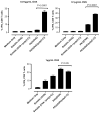
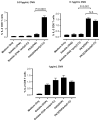
In vitro DC-OT-1 cross-priming by soluble and PK3 microparticle vaccine formulations containing OVA or (OVA + poly(I:C)); percentage of IFNγ-producing CD8+ T cells (n = 4 wells per group). Data shown above is one representative experiment out of two independent experiments with similar trends.
Fig. 8
In vitro DC-OT-1 cross-priming by soluble and PK3 microparticle vaccine formulations containing OVA or (OVA + poly(I:C)); percentage of TNFα-producing CD8+ T cells (n = 4 wells per group). Data shown above is one representative experiment out of two independent experiments with similar trends.
Fig. 9
In vitro DC-OT-1 cross-priming by soluble and PK3 microparticle vaccine formulations containing OVA or (OVA + poly(I:C)); percentage of IL-2-producing CD8+ T cells (n = 4 wells per group). Data shown above is one representative experiment out of two independent experiments with similar trends.
Similar articles
- Particulate formulations for the delivery of poly(I:C) as vaccine adjuvant.
Hafner AM, Corthésy B, Merkle HP. Hafner AM, et al. Adv Drug Deliv Rev. 2013 Oct;65(10):1386-99. doi: 10.1016/j.addr.2013.05.013. Epub 2013 Jun 7. Adv Drug Deliv Rev. 2013. PMID: 23751781 Review. - Cationic liposomes loaded with a synthetic long peptide and poly(I:C): a defined adjuvanted vaccine for induction of antigen-specific T cell cytotoxicity.
Varypataki EM, van der Maaden K, Bouwstra J, Ossendorp F, Jiskoot W. Varypataki EM, et al. AAPS J. 2015 Jan;17(1):216-26. doi: 10.1208/s12248-014-9686-4. Epub 2014 Nov 12. AAPS J. 2015. PMID: 25387996 Free PMC article. - A Highly Active Form of XCL1/Lymphotactin Functions as an Effective Adjuvant to Recruit Cross-Presenting Dendritic Cells for Induction of Effector and Memory CD8+ T Cells.
Matsuo K, Kitahata K, Kawabata F, Kamei M, Hara Y, Takamura S, Oiso N, Kawada A, Yoshie O, Nakayama T. Matsuo K, et al. Front Immunol. 2018 Nov 27;9:2775. doi: 10.3389/fimmu.2018.02775. eCollection 2018. Front Immunol. 2018. PMID: 30542351 Free PMC article. - Encapsulation of antigen in poly(D,L-lactide-co-glycolide) microspheres protects from harmful effects of γ-irradiation as assessed in mice.
Mohanan D, Gander B, Kündig TM, Johansen P. Mohanan D, et al. Eur J Pharm Biopharm. 2012 Feb;80(2):274-81. doi: 10.1016/j.ejpb.2011.10.007. Epub 2011 Oct 18. Eur J Pharm Biopharm. 2012. PMID: 22024408 - In situ engineering of the lymph node microenvironment via intranodal injection of adjuvant-releasing polymer particles.
Jewell CM, López SC, Irvine DJ. Jewell CM, et al. Proc Natl Acad Sci U S A. 2011 Sep 20;108(38):15745-50. doi: 10.1073/pnas.1105200108. Epub 2011 Sep 6. Proc Natl Acad Sci U S A. 2011. PMID: 21896725 Free PMC article.
Cited by
- A VLP-Based Vaccine Displaying HBHA and MTP Antigens of Mycobacterium tuberculosis Induces Protective Immune Responses in M. tuberculosis H37Ra Infected Mice.
Wang J, Xie T, Ullah I, Mi Y, Li X, Gong Y, He P, Liu Y, Li F, Li J, Lu Z, Zhu B. Wang J, et al. Vaccines (Basel). 2023 May 4;11(5):941. doi: 10.3390/vaccines11050941. Vaccines (Basel). 2023. PMID: 37243045 Free PMC article. - Magnetic nanoparticles enhance the cellular immune response of dendritic cell tumor vaccines by realizing the cytoplasmic delivery of tumor antigens.
Huang L, Liu Z, Wu C, Lin J, Liu N. Huang L, et al. Bioeng Transl Med. 2022 Sep 9;8(2):e10400. doi: 10.1002/btm2.10400. eCollection 2023 Mar. Bioeng Transl Med. 2022. PMID: 36925683 Free PMC article. - Hydrophobic ion pairing: encapsulating small molecules, peptides, and proteins into nanocarriers.
Ristroph KD, Prud'homme RK. Ristroph KD, et al. Nanoscale Adv. 2019 Oct 1;1(11):4207-4237. doi: 10.1039/c9na00308h. Nanoscale Adv. 2019. PMID: 33442667 Free PMC article. Review. - Modular Polymer Antigens To Optimize Immunity.
Bennett NR, Jarvis CM, Alam MM, Zwick DB, Olson JM, Nguyen HV, Johnson JA, Cook ME, Kiessling LL. Bennett NR, et al. Biomacromolecules. 2019 Dec 9;20(12):4370-4379. doi: 10.1021/acs.biomac.9b01049. Epub 2019 Nov 11. Biomacromolecules. 2019. PMID: 31609600 Free PMC article. - Gold nanoparticles conjugating recombinant influenza hemagglutinin trimers and flagellin enhanced mucosal cellular immunity.
Wang C, Zhu W, Luo Y, Wang BZ. Wang C, et al. Nanomedicine. 2018 Jun;14(4):1349-1360. doi: 10.1016/j.nano.2018.03.007. Epub 2018 Apr 9. Nanomedicine. 2018. PMID: 29649593 Free PMC article.
References
- van Duin D, Medzhitov R, Shaw AC. Triggering TLR signaling in vaccination. Trends Immunol. 2006;27(1):49–55. - PubMed
- Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8(4):247–58. - PubMed
- Pashine A, Valiante NM, Ulmer JB. Targeting the innate immune response with improved vaccine adjuvants. Nat Med. 2005;11(4 Suppl):S63–8. - PubMed
- Heit A, Busch DH, Wagner H, Schmitz F. Vaccine protocols for enhanced immunogenicity of exogenous antigens. Int J Med Microbiol. 2008;298(1–2):27–32. - PubMed
- Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5(10):987–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 HL080711/HL/NHLBI NIH HHS/United States
- U19 AI057266/AI/NIAID NIH HHS/United States
- R01 DK057665/DK/NIDDK NIH HHS/United States
- N01 A1-50019/PHS HHS/United States
- N01 A150025/PHS HHS/United States
- R01 AI056499/AI/NIAID NIH HHS/United States
- R01 AI056499-01/AI/NIAID NIH HHS/United States
- R01 AI048638/AI/NIAID NIH HHS/United States
- U01 HL80711-01/HL/NHLBI NIH HHS/United States
- U54 AI057157/AI/NIAID NIH HHS/United States
- R01 AI064463/AI/NIAID NIH HHS/United States
- R01 AI48638-01/AI/NIAID NIH HHS/United States
- R01 DK 57665-01/DK/NIDDK NIH HHS/United States
- R21 EB006418/EB/NIBIB NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous