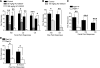Transient receptor potential ankyrin 1 antagonists block the noxious effects of toxic industrial isocyanates and tear gases - PubMed (original) (raw)
Transient receptor potential ankyrin 1 antagonists block the noxious effects of toxic industrial isocyanates and tear gases
Bret F Bessac et al. FASEB J. 2009 Apr.
Abstract
The release of methyl isocyanate in Bhopal, India, caused the worst industrial accident in history. Exposures to industrial isocyanates induce lacrimation, pain, airway irritation, and edema. Similar responses are elicited by chemicals used as tear gases. Despite frequent exposures, the biological targets of isocyanates and tear gases in vivo have not been identified, precluding the development of effective countermeasures. We use Ca(2+) imaging and electrophysiology to show that the noxious effects of isocyanates and those of all major tear gas agents are caused by activation of Ca(2+) influx and membrane currents in mustard oil-sensitive sensory neurons. These responses are mediated by transient receptor potential ankyrin 1 (TRPA1), an ion channel serving as a detector for reactive chemicals. In mice, genetic ablation or pharmacological inhibition of TRPA1 dramatically reduces isocyanate- and tear gas-induced nocifensive behavior after both ocular and cutaneous exposures. We conclude that isocyanates and tear gas agents target the same neuronal receptor, TRPA1. Treatment with TRPA1 antagonists may prevent and alleviate chemical irritation of the eyes, skin, and airways and reduce the adverse health effects of exposures to a wide range of toxic noxious chemicals.
Figures
Figure 1.
Industrial isocyanates and tear gases activate heterologously expressed TRPA1 channels. A) Chemical structures of environmental and occupational irritants MITC, MIC, and HDI. B) Structures of tear gas agents CN, CS, CR, benzyl bromide and bromoacetone (bromo-2-propanone), and PS. C) Dose-response curves of isocyanate-activated Ca2+ influx into hTRPA1-transfected HEK-293T cells. [Ca2+]i induced by each dose is represented as percentage of maximal [Ca2+]i elicited by a saturating dose of mustard oil (100 μM) applied 75 s later (baseline [Ca2+]i was subtracted). hTRPA1 was activated by MIC (EC50=25±3 μM, _n_=28±5 cells/dose, red squares) and HDI (EC50=2.6±0.7 μM, _n_=29±4 cells/dose, black circles). D) Dose-response analysis of tear gas agent-activated Ca2+ influx into hTRPA1-transfected HEK-293T cells. [Ca2+]i represented as in A. hTRPA1 was activated by CN (EC50=91±12 nM, _n_=49±1 cells/dose, green triangles), CS (EC50=7±1 nM, _n_=52±5, blue squares), CR (EC50=308±150 nM, _n_=70±9, red circles), PS (EC50=215±62 nM, _n_=32±4, gray diamonds), benzyl bromide (BenzBr, EC50=12±1 μM, _n_=66±17, black triangles) and bromoacetone (BrAc, EC50=1.1±0.3 μM, _n_=78±14 cells/dose, purple stars). E) Application of MIC (100 μM, purple bar) induces an increase of single-channel openings of an excised patch in the inside-out configuration from hTRPA1-overexpressing CHO cells. Voltage was held at −40 mV; bath solution contained 0.5 mM PPPi and was devoid of Ca2+ (10 mM EGTA). F) Application of CS (10 μM, blue bar) induces an increase of single-channel openings in an excised patch in the inside-out configuration from hTRPA1-overexpressing CHO cells. Conditions were same as for E. G) Responses of hTRPA1 mutant-expressing HEK-293T cells to 100 μM MIC, 100 μM HDI, 100 μM CS, 100 μM CN, 300 μM CR, 100 μM bromoacetone (BrAc), and 100 μM benzyl bromide (BenzBr). Increase in [Ca2+]i is displayed as percentage of [Ca2+]i activated by a saturating dose of carvacrol (300 μM). Mutants tested are coded as follows: K (gray) = K708 (MIC, _n_=84; HDI, _n_=53; CS, _n_=157; CN, _n_=108; CR, _n_=154; BrAc, _n_=44; BenzBr _n_=64 cells); 3C (white) = C619, C639, and C663 combined (MIC, _n_=34; HDI, _n_=47; CS, _n_=96; CN, _n_=56; CR, _n_=90; BrAc, _n_=25; BenzBr, _n_=40 cells); 3CK (black) = C619, C639, and C663; and K708 combined (MIC, _n_=30; HDI, _n_=123; CS, _n_=129; CN, _n_=18; CR, _n_=17; BrAc, _n_=38; BenzBr, _n_=41).
Figure 2.
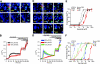
Industrial isocyanates and tear gas agents activate native TRPA1 channels in cultured sensory neurons. A) Industrial isocyanates induced Ca2+ influx into cultured mouse DRG neurons, as measured by fluorescent Fura-2 imaging. Neurons are shown before activation (Pre, left column), 70 s after challenge (middle column) with MIC (100 μM, top row) or HDI (100 μM, bottom row), and after application of 5 μM capsaicin (Cap, right column) after 50 s. Pseudocolors denote 0–3 μM [Ca2+]i. Original view, ×10. B) Average [Ca2+]i of mouse DRG neurons (thick lines) with an application of MIC (100 μM, _n_=168 neurons from 2 mice, black line) or HDI (100 μM, _n_=270 from 2 mice, red line), followed by 100 μM mustard oil, 5 μM capsaicin (Cap), and 65 mM KCl. Thin lines represent
se
. C) Tear gas agent-induced Ca2+ influx into cultured murine DRG neurons, as measured by fluorescent Fura-2 imaging. Neurons are shown before activation (Pre, left column), 70 s after challenge (middle column) with CS (100 μM, top row) or CN (100 μM, middle row) or CR (300 μM, bottom row) and after application of 5 μM capsaicin (Cap, right column) after 50 s. Pseudocolors denote 0–3 μM [Ca2+]i. Original view, ×10. D) Average [Ca2+]i of mouse DRG neurons (thick lines) with an application of CS (100 μM, blue line, _n_=161 neurons from 2 mice), CN (100 μM, green line, _n_=335 from 5 mice), or CR (300 μM, red line, _n_=137 from 2 mice), followed by 100 μM mustard oil, 5 μM capsaicin (Cap), and 65 mM KCl. Thin lines represent
se
. E) Dose-response curves of isocyanate-activated Ca2+-influx into mouse DRG neurons are similar to hTRPA1-transfected HEK-293T cells. [Ca2+]i induced by each dose is represented as percentage of maximal [Ca2+]i elicited by a saturating dose of mustard oil (100 μM) applied 75 s later (baseline [Ca2+]i was subtracted). Mustard oil-sensitive mouse DRG neurons were activated by MIC (EC50=36±7 μM, _n_=30±6 neurons/dose, solid black squares) and HDI (EC50=8.4±1.4 μM, n_=39±12 /dose, solid red squares). Dashed lines and open circles represent hTRPA1-transfected HEK-293T cells, as shown in Fig. 1_C. F) Dose-response curves of tear gas agent-activated Ca2+-influx into mouse DRG neurons are right-shifted compared with responses in hTRPA1-transfected HEK293T cells. [Ca2+]i represented as in E. Mustard oil-sensitive mouse DRG neurons were activated by CS (EC50=12.1±0.3 μM, _n_=41±9 neurons/dose, solid blue squares), CN (EC50 =6±1 μM, _n_=23±5 /dose, solid green squares), and CR (EC50= 246±27 μM, n_=37±16 /dose, solid red squares). Dashed lines with open circles represent dose-response curves of Ca2+-influx into hTRPA1-transfected HEK-293T cells shown in Fig. 1_D.
Figure 3.
CN induces TRPA1-like currents in mouse DRG neurons. A) TRPA1-like current-voltage curves of a representative mouse DRG neuron before activation (black trace), activation by 100 μM CN (green trace), and inhibition by ruthenium red (10 μM, red trace) in whole-cell configuration. V_holding = 0 mV to minimize voltage-gated channels. Currents were measured with a voltage ramp from −100 to +100 mV over 100 ms at 0.5-Hz intervals. Intracellular Cs-based solution with 10 mM EGTA was used. B) Average native TRPA1-like currents at −80 and +80 mV in mouse DRG neurons superfused with 100 μM CN (black bar), followed by ruthenium red (RuRed; 10 μM) as described for Fig. 1_B (n_=4 of 16 neurons). Baseline current was subtracted for each trace. C) hTRPA1 current-voltage curves before activation (black trace), at maximal activation by 10 μM CN (green trace), and after inactivation phase (blue trace) in whole-cell configuration. Currents were measured with a voltage ramp from −80 to +80 mV over 100 ms at 0.5 Hz intervals, Vholding= 0 mV. Intracellular Cs-based solution with 10 mM EGTA was used. D) Averaged TRPA1 currents at −80 and +80 mV in hTRPA1-transfected HEK-293T cells superfused with 10 μM CN (black bar) as described for Fig. 1_C (_n_=4).
Figure 4.
Ablation of isocyanate- and tear gas agent-induced sensory neuronal activation by genetic ablation or pharmacological blockade of TRPA1. A) Isocyanate-induced Ca2+ influx is absent in DRG neurons from _Trpa1_−/− mice. Neurons are shown before application (Pre, left column), 70 s after challenge (middle column) with MIC (100 μM, top row) or HDI (100 μM, bottom row), and after 5 μM capsaicin (Cap, right column) after 50 s. Pseudocolors denote 0–3 μM [Ca2+]i. Original view, ×10. B) Average [Ca2+]i of mouse DRG neurons (thick lines) with an application of MIC (100 μM, red line, _n_=217 from 2 mice) and HDI (100 μM, black line, _n_=204 neurons from 2 mice), followed by 100 μM mustard oil, 5 μM capsaicin (Cap), and 65 mM KCl. Thin lines represent
se
. C) Tear gas agent-induced Ca2+ influx is absent in DRG neurons from _Trpa1_−/− mice, shown before activation (Pre, left column), 70 s after challenge (middle column) with CS (100 μM, top row), CN (100 μM, middle row), or CR (300 μM, bottom row), followed by 5 μM capsaicin (Cap, right column) after 50 s. Pseudocolors denote 0–3 μM [Ca2+]i. Original view, ×10. D) Average [Ca2+]i of mouse DRG neurons (thick lines) with an application of CS (10 μM, blue line, _n_=229 neurons from 2 mice), CN (100 μM, green line, _n_=270 neurons from 5 mice), or CR (300 μM, red line, _n_=108 neurons), followed by 100 μM mustard oil, 5 μM capsaicin (Cap), and 65 mM KCl. Thin lines represent
se
. E) Dose-response curves of inhibition of industrial isocyanate or tear gas agent-activated Ca2+-influx into mouse DRG neurons by the TRPA1 antagonist HC-030031. [Ca2+]i induced by each dose is represented as percentage of [Ca2+]i elicited by a saturating dose of capsaicin (5 μM, Cap) applied 125 s later (baseline [Ca2+]i was subtracted). HC-030031 inhibited the [Ca2+]i induced by 10 μM HDI (IC50=74±3 μM, _n_=31±4 Cap-sensitive neurons/dose, black triangles), 10 μM CS (IC50=4.5±0.4 μM, _n_=26±6/ dose, blue squares), and 10 μM CN (IC50=884±23 nM, _n_=25±5/dose, green circles) in mouse DRG neurons.
Figure 5.
Ablation of isocyanate- and tear gas agent-induced nocifensive responses in mice by genetic deletion or pharmacological blockade of TRPA1. A) Nocifensive responses after application of 10 μl of 100 mM HDI (_n_=6), 100 mM CS (_n_=6), or 100 mM CN (_n_=9) to the eye of untreated C57/BL6 wild-type mice (black bars) and the same mice after an injection of 1 mg i.p.(50 mg/kg, gray bar) or 6 mg i.p.(300 mg/kg, white bar) of TRPA1 antagonist HC-030031. Nocifensive responses were quantified by counting strokes of the orbitofacial area on the observation chamber floor over 2 min for CS and CN for 300 mg/kg experiments and over 3 min for other treatments. Significance: **P < 0.01; *P < 0.05. B) Nocifensive responses (licks, lifts, and flicks) over 3 min after 25-μl subplantar injections of 4 mM CN or 6 mM HDI (_n_=6/group) into hindpaws of untreated C57/BL6 wild-type mice (black bars) and the same mice after an injection of 2 mg i.p.(100 mg/kg, gray bars). Significance: **P < 0.01; *P < 0.05. C) Comparison of nocifensive responses of wild-type (black bars) and _Trpa1_−/− mice (white bars) after application of 10 μl of 200 mM HDI, 100 mM CS, or 100 mM CN to right eye. Strokes of the orbitofacial area against the observation chamber floor were counted over 2 min for CS and CN and for 3 min for HDI (_n_=6 wild-type and _n_=6 _Trpa1_−/− mice were tested with CN, _n_=6 wild-type and _n_=7 _Trpa1_−/− mice tested with CS, and _n_=6 wild-type and _n_=7 _Trpa1_−/− mice were tested with HDI). ***P < 0.001; **P < 0.01; *P < 0.05. D) Nocifensive responses (licks, lifts, and flicks) over 5 min after 25-μl subplantar injections of 2 mM CN (_n_=8/group) or 4 mM bromoacetone (Br acetone, _n_=6/group) into hindpaws of Trpa1+/+ and _Trpa1_−/− mice. *P < 0.05.
Comment in
- Regarding "Transient receptor potential ankyrin 1 antagonists block the noxious effects of toxic industrial isocyanates and tear gases".
Shusterman D. Shusterman D. FASEB J. 2010 Apr;24(4):980. doi: 10.1096/fj.10-0405LTR. FASEB J. 2010. PMID: 20356910 No abstract available.
Similar articles
- Regarding "Transient receptor potential ankyrin 1 antagonists block the noxious effects of toxic industrial isocyanates and tear gases".
Shusterman D. Shusterman D. FASEB J. 2010 Apr;24(4):980. doi: 10.1096/fj.10-0405LTR. FASEB J. 2010. PMID: 20356910 No abstract available. - Cannabinoids desensitize capsaicin and mustard oil responses in sensory neurons via TRPA1 activation.
Akopian AN, Ruparel NB, Patwardhan A, Hargreaves KM. Akopian AN, et al. J Neurosci. 2008 Jan 30;28(5):1064-75. doi: 10.1523/JNEUROSCI.1565-06.2008. J Neurosci. 2008. PMID: 18234885 Free PMC article. - TRPA1-mediated responses in trigeminal sensory neurons: interaction between TRPA1 and TRPV1.
Salas MM, Hargreaves KM, Akopian AN. Salas MM, et al. Eur J Neurosci. 2009 Apr;29(8):1568-78. doi: 10.1111/j.1460-9568.2009.06702.x. Eur J Neurosci. 2009. PMID: 19419422 Free PMC article. - Sensory detection and responses to toxic gases: mechanisms, health effects, and countermeasures.
Bessac BF, Jordt SE. Bessac BF, et al. Proc Am Thorac Soc. 2010 Jul;7(4):269-77. doi: 10.1513/pats.201001-004SM. Proc Am Thorac Soc. 2010. PMID: 20601631 Free PMC article. Review. - Increasingly irritable and close to tears: TRPA1 in inflammatory pain.
McMahon SB, Wood JN. McMahon SB, et al. Cell. 2006 Mar 24;124(6):1123-5. doi: 10.1016/j.cell.2006.03.006. Cell. 2006. PMID: 16564004 Review.
Cited by
- Transient receptor potential channels as therapeutic targets.
Moran MM, McAlexander MA, Bíró T, Szallasi A. Moran MM, et al. Nat Rev Drug Discov. 2011 Aug 1;10(8):601-20. doi: 10.1038/nrd3456. Nat Rev Drug Discov. 2011. PMID: 21804597 Review. - Transient receptor potential (TRP) channels as drug targets for diseases of the digestive system.
Holzer P. Holzer P. Pharmacol Ther. 2011 Jul;131(1):142-70. doi: 10.1016/j.pharmthera.2011.03.006. Epub 2011 Mar 21. Pharmacol Ther. 2011. PMID: 21420431 Free PMC article. Review. - An Official American Thoracic Society Workshop Report: Chemical Inhalational Disasters. Biology of Lung Injury, Development of Novel Therapeutics, and Medical Preparedness.
Summerhill EM, Hoyle GW, Jordt SE, Jugg BJ, Martin JG, Matalon S, Patterson SE, Prezant DJ, Sciuto AM, Svendsen ER, White CW, Veress LA; ATS Terrorism and Inhalational Disasters Section of the Environmental, Occupational, and Population Health Assembly. Summerhill EM, et al. Ann Am Thorac Soc. 2017 Jun;14(6):1060-1072. doi: 10.1513/AnnalsATS.201704-297WS. Ann Am Thorac Soc. 2017. PMID: 28418689 Free PMC article. - Noxious effects of riot control agents on the ocular surface: Pathogenic mechanisms and management.
Quiroga-Garza ME, Ruiz-Lozano RE, Azar NS, Mousa HM, Komai S, Sevilla-Llorca JL, Perez VL. Quiroga-Garza ME, et al. Front Toxicol. 2023 Jan 17;5:1118731. doi: 10.3389/ftox.2023.1118731. eCollection 2023. Front Toxicol. 2023. PMID: 36733462 Free PMC article. Review. - Alleviation of methyl isocyanate-induced airway obstruction and mortality by tissue plasminogen activator.
Nick HJ, Rioux JS, Veress LA, Bratcher PE, Bloomquist LA, Anantharam P, Croutch CR, Tuttle RS, Peters E, Sosna W, White CW. Nick HJ, et al. Ann N Y Acad Sci. 2020 Nov;1479(1):134-147. doi: 10.1111/nyas.14344. Epub 2020 Mar 31. Ann N Y Acad Sci. 2020. PMID: 32233099 Free PMC article.
References
- Dhara V R, Dhara R. The Union Carbide disaster in Bhopal: a review of health effects. Arch Environ Health. 2002;57:391–404. - PubMed
- Koehler G A, Van Ness C. The emergency medical response to the Cantara hazardous materials incident. Prehosp Disaster Med. 1993;8:359–365. - PubMed
- Pruett S B, Myers L P, Keil D E. Toxicology of metam sodium. J Toxicol Environ Health B Crit Rev. 2001;4:207–222. - PubMed
- Dermatitis among workers cleaning the Sacramento River after a chemical spill—California, 1991. MMWR Morb Mortal Wkly Rep. 1991;40:825–827. 833. - PubMed
- Cone J E, Wugofski L, Balmes J R, Das R, Bowler R, Alexeeff G, Shusterman D. Persistent respiratory health effects after a metam sodium pesticide spill. Chest. 1994;106:500–508. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U54 ES017218/ES/NIEHS NIH HHS/United States
- F31ES015932/ES/NIEHS NIH HHS/United States
- U01 ES015674/ES/NIEHS NIH HHS/United States
- F31 ES015932/ES/NIEHS NIH HHS/United States
- R01 ES015056/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous