Identification of a receptor required for the anti-inflammatory activity of IVIG - PubMed (original) (raw)
Identification of a receptor required for the anti-inflammatory activity of IVIG
Robert M Anthony et al. Proc Natl Acad Sci U S A. 2008.
Abstract
The anti-inflammatory activity of intravenous Ig (IVIG) results from a minor population of the pooled IgG molecules that contains terminal alpha2,6-sialic acid linkages on their Fc-linked glycans. These anti-inflammatory properties can be recapitulated with a fully recombinant preparation of appropriately sialylated IgG Fc fragments. We now demonstrate that these sialylated Fcs require a specific C-type lectin, SIGN-R1, (specific ICAM-3 grabbing non-integrin-related 1) expressed on macrophages in the splenic marginal zone. Splenectomy, loss of SIGN-R1(+) cells in the splenic marginal zone, blockade of the carbohydrate recognition domain (CRD) of SIGN-R1, or genetic deletion of SIGN-R1 abrogated the anti-inflammatory activity of IVIG or sialylated Fc fragments. Although SIGN-R1 has not previously been shown to bind to sialylated glycans, we demonstrate that it preferentially binds to 2,6-sialylated Fc compared with similarly sialylated, biantennary glycoproteins, thus suggesting that a specific binding site is created by the sialylation of IgG Fc. A human orthologue of SIGN-R1, DC-SIGN, displays a similar binding specificity to SIGN-R1 but differs in its cellular distribution, potentially accounting for some of the species differences observed in IVIG protection. These studies thus identify an antibody receptor specific for sialylated Fc, and present the initial step that is triggered by IVIG to suppress inflammation.
Conflict of interest statement
Conflict of interest statement: J.V.R is a founder and shareholder of Virdante Pharmaceutical, Inc.
Figures
Fig. 1.
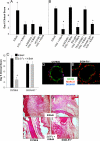
A splenic macrophage population is required for IVIG anti-inflammatory activity. Wild-type C57BL/6 mice, mice deficient in B cells (JHD−/−), mice deficient in CD4+ T cells (CD4−/−), mice deficient in both B and T cells (Rag1−/−), and splenectomized mice were treated with IVIG and K/BxN sera, and footpad swelling was monitored. Means and standard deviation of day 6 clinical scores from 4–5 mice per group are plotted, and are representative of three separate experiments; *, P < 0.05 as determined by a Student t test.
Fig. 2.
The marginal zone macrophage receptor SIGN-R1 is required for IVIG anti-inflammatory activity. (A) Mice were administered blocking antibodies to marginal zone receptors CD169 (α-CD169), and MARCO (α-MARCO), treated with IVIG followed by K/BxN sera, and footpad swelling monitored. Mean and standard deviation of day 6 clinical scores from 4–5 mice per group are plotted; *, P < 0.05 as determined by ANOVA followed by a Tukey post-hoc test. (B) In a separate experiment, mice were treated with SIGN-R1 blocking antibodies (α-SIGNR1), isotype control antibodies for α-SIGNR1 (Rat IgM), antibodies that transiently knockdown SIGN-R1 expression (TKO-SIGN-R1), or an appropriate isotype control for TKO-SIGN-R1 (Hamster IgG). Next, IVIG and K/BxN were administered, and footpad swelling was monitored over the following week; means and standard deviations of 4–5 mice per group are plotted. *, P < 0.05 as determined by Anova followed by Tukey post-hoc. (C) C57BL/6 and SIGN-R1−/− mice were administered arthritis inducing sera (K/BxN, black bars), some of which received 2,6-Fcs 1 h earlier (2,6-Fc + K/BxN, gray bars). Footpad swelling was monitored over the next seven days in terms of clinical scores. Means and standard deviations of 34 mice per group are plotted. *, P < 0.05 as determined by ANOVA followed by Tukey post-hoc. The splenic marginal zones of C57BL/6 wild type (D) and SIGN-R1−/− (E) mice were examined by immunofluorescence for CD169 (green), MARCO (red), and SIGN-R1 (blue). Monocyte and neutrophil infiltration in the ankle bones of C57BL/6 or SIGN-R1−/− mice were examined in H&E-stained sections 7 days after treatment. Darkly stained nuclei of monocytes and neutrophils infiltrate the ankle bones of both C57BL/6 (F) and SIGN-R1−/− (G) mice treated with K/BxN sera; the inflammatory infiltration is reduced in C57BL/6 mice receiving 2,6-Fc treatment (H) but not in 2,6-Fc-treated SIGN-R1−/− (I) mice.
Fig. 3.
Op/op mice lack SIGN-R1 expression on marginal zone macrophages. Spleen sections from wild type C57BL/6 (A) and op/op mice (B) were examined for marginal zone macrophage receptor expression by immunofluorescent staining for CD169 (green), MARCO (red), and SIGN-R1 (blue). Imaging conditions for each fluorescent channel were identical for each sample.
Fig. 4.
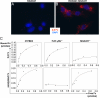
SIGN-R1 expressing cells preferentially bind 2,6-sialylated Fc's. RAW (A) and RAW-SIGN-R1 cells (B) were pulsed with fluorochrome-labeled 2,6-Fcs (red), stained with DAPI (blue), and imaged at 400× with identical exposure times and intensities. (C) Resident peritoneal macrophages isolated from C57BL/6 (left column), FcR γ/IIb−/− (middle column), and SIGN-R1−/− (right column) mice were pulsed with increasing concentrations of 2,6-sialylated Fcs (top row) or asialylated Fcs (bottom row). The amount of bound Fcs were determined and are plotted verses the free, unbound Fcs, and are representative of two separate experiments.
Fig. 5.
Human DC-SIGN and SIGN-R1 display similar binding profiles of sialylated Fcs. (A) Amino acid sequences of the carbohydrate recognition domains (CRD) of human DC-SIGN and SIGN-R1 are aligned. Yellow and green highlights indicate identical and similar amino acids, respectively. (B) CHO cells expressing SIGN-R1 (left column), hDC-SIGN (middle column), or hFcγRIIb (right column) were pulsed with 2,6-Fcs (top row), incubated with mannan before 2,6-Fc pulse (middle row), or pulsed with fibrinogen (bottom row). The amount of bound glycoproteins was determined, and is plotted verses the free, unbound protein. _K_a values determined by linear regression analysis are displayed in Table 1.
Fig. 6.
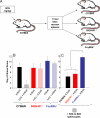
IVIG treated splenocytes can transfer anti-inflammatory activity, but require inhibitory FcγRIIb expression in recipient mice. (A) A schematic diagram of an IVIG-adoptive transfer system, where C57BL/6 mice are administered IVIG, killed 1 h later, splenocytes recovered and administered to recipient C57BL/6, SIGN-R1−/−, FcγRIIb−/− mice, that are subsequently given K/BxN sera. (B) C57BL/6 (black), SIGN-R1−/− (red), and FcγRIIb−/− (blue) mice were administered K/BxN (solid bars) or IVIG and K/BxN (hatched bars), and footpad swelling monitored. (C) In parallel, IVIG treated splenocytes from C57BL/6 mice were transferred to C57BL/6 (black), SIGN-R1−/− (red), and FcγRIIb−/− (blue) mice, which were administered K/BxN and footpad swelling monitored. Clinical scores of 4–5 mice per group four days after treatment are plotted; P < 0.05 as determined by ANOVA followed by Tukey post-hoc test.
Fig. 7.
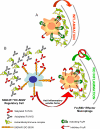
A model for the anti-inflammatory activity of 2,6-sialylated Fc. Features of this model have been presented previously. (A) Under inflammatory and autoimmune conditions, immune complexes formed by autoantigens and self-reactive antibodies bind activating FcR expressed by inflammatory macrophages, leading to their activation. (B) 2,6-Sialylated Fc, found in preparations of i.v. gamma globulin (IVIG), engages a lectin on the surface of a regulatory macrophage population in the spleen found in the marginal zone, now identified as SIGN-R1. Engagement of this lectin induces a cellular program that results in the secretion of anti-inflammatory, soluble mediators that target effector macrophages found at the site of tissue inflammation where pathogenic immune complexes are deposited. These effector macrophages respond to the anti-inflammatory mediators by increasing surface expression of the inhibitory FcgRIIB receptor, thereby altering the threshold concentration of immune complexes necessary to trigger macrophage activation and subsequent inflammation. The net result of this pathway then is to attenuate autoantibody mediated inflammation and tissue pathology. The homologous pathway in the human differs in that the lectin is DC-SIGN and the regulatory cells are dendritic cells and thus, not restricted to the spleen.
Comment in
- DC-SIGN and alpha2,6-sialylated IgG Fc interaction is dispensable for the anti-inflammatory activity of IVIg on human dendritic cells.
Bayry J, Bansal K, Kazatchkine MD, Kaveri SV. Bayry J, et al. Proc Natl Acad Sci U S A. 2009 Mar 3;106(9):E24; author reply E25. doi: 10.1073/pnas.0900016106. Epub 2009 Feb 23. Proc Natl Acad Sci U S A. 2009. PMID: 19237553 Free PMC article. No abstract available. - Literature Watch: implications for transplantation.
Turnquist HR, Bromberg JS, Bromberg JS. Turnquist HR, et al. Am J Transplant. 2012 Oct;12(10):2567. doi: 10.1111/j.1600-6143.2012.04297.x. Am J Transplant. 2012. PMID: 23009135 No abstract available.
Similar articles
- Dissecting the molecular mechanism of IVIg therapy: the interaction between serum IgG and DC-SIGN is independent of antibody glycoform or Fc domain.
Yu X, Vasiljevic S, Mitchell DA, Crispin M, Scanlan CN. Yu X, et al. J Mol Biol. 2013 Apr 26;425(8):1253-8. doi: 10.1016/j.jmb.2013.02.006. Epub 2013 Feb 13. J Mol Biol. 2013. PMID: 23416198 - DC-SIGN and alpha2,6-sialylated IgG Fc interaction is dispensable for the anti-inflammatory activity of IVIg on human dendritic cells.
Bayry J, Bansal K, Kazatchkine MD, Kaveri SV. Bayry J, et al. Proc Natl Acad Sci U S A. 2009 Mar 3;106(9):E24; author reply E25. doi: 10.1073/pnas.0900016106. Epub 2009 Feb 23. Proc Natl Acad Sci U S A. 2009. PMID: 19237553 Free PMC article. No abstract available. - A novel role for the IgG Fc glycan: the anti-inflammatory activity of sialylated IgG Fcs.
Anthony RM, Ravetch JV. Anthony RM, et al. J Clin Immunol. 2010 May;30 Suppl 1:S9-14. doi: 10.1007/s10875-010-9405-6. J Clin Immunol. 2010. PMID: 20480216 Review. - Intravenous gammaglobulin suppresses inflammation through a novel T(H)2 pathway.
Anthony RM, Kobayashi T, Wermeling F, Ravetch JV. Anthony RM, et al. Nature. 2011 Jun 19;475(7354):110-3. doi: 10.1038/nature10134. Nature. 2011. PMID: 21685887 Free PMC article. - Intravenous immunoglobulin therapy: how does IgG modulate the immune system?
Schwab I, Nimmerjahn F. Schwab I, et al. Nat Rev Immunol. 2013 Mar;13(3):176-89. doi: 10.1038/nri3401. Epub 2013 Feb 15. Nat Rev Immunol. 2013. PMID: 23411799 Review.
Cited by
- Mutation of Y407 in the CH3 domain dramatically alters glycosylation and structure of human IgG.
Rose RJ, van Berkel PH, van den Bremer ET, Labrijn AF, Vink T, Schuurman J, Heck AJ, Parren PW. Rose RJ, et al. MAbs. 2013 Mar-Apr;5(2):219-28. doi: 10.4161/mabs.23532. Epub 2013 Feb 13. MAbs. 2013. PMID: 23406897 Free PMC article. - Macrophage heterogeneity in lymphoid tissues.
den Haan JM, Martinez-Pomares L. den Haan JM, et al. Semin Immunopathol. 2013 Sep;35(5):541-52. doi: 10.1007/s00281-013-0378-4. Epub 2013 Apr 12. Semin Immunopathol. 2013. PMID: 23579230 - Intravenous immunoglobulins modulate neutrophil activation and vascular injury through FcγRIII and SHP-1.
Jang JE, Hidalgo A, Frenette PS. Jang JE, et al. Circ Res. 2012 Apr 13;110(8):1057-66. doi: 10.1161/CIRCRESAHA.112.266411. Epub 2012 Mar 13. Circ Res. 2012. PMID: 22415018 Free PMC article. - In vitro and in vivo studies of IgG-derived Treg epitopes (Tregitopes): a promising new tool for tolerance induction and treatment of autoimmunity.
Cousens LP, Najafian N, Mingozzi F, Elyaman W, Mazer B, Moise L, Messitt TJ, Su Y, Sayegh M, High K, Khoury SJ, Scott DW, De Groot AS. Cousens LP, et al. J Clin Immunol. 2013 Jan;33 Suppl 1(Suppl 1):S43-9. doi: 10.1007/s10875-012-9762-4. Epub 2012 Sep 2. J Clin Immunol. 2013. PMID: 22941509 Free PMC article. Review. - Comparison of the Fc glycosylation of fetal and maternal immunoglobulin G.
Einarsdottir HK, Selman MH, Kapur R, Scherjon S, Koeleman CA, Deelder AM, van der Schoot CE, Vidarsson G, Wuhrer M. Einarsdottir HK, et al. Glycoconj J. 2013 Feb;30(2):147-57. doi: 10.1007/s10719-012-9381-6. Epub 2012 May 10. Glycoconj J. 2013. PMID: 22572841 Free PMC article.
References
- Ravetch JV, Nimmerjahn F. In: Fundamental Immunology. Paul WE, editor. Philadelphia: Lippincott Williams and Wilkins; 2007. pp. 684–705.
- Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. - PubMed
- Nimmerjahn F, Ravetch JV. Anti-inflammatory actions of intravenous immunoglobulin. Annu Rev Immunol. 2008;26:513–533. - PubMed
- Clynes R. Protective mechanisms of IVIG. Curr Opin Immunol. 2007;19:646–651. - PubMed
- Negi VS, et al. Intravenous immunoglobulin: An update on the clinical use and mechanisms of action. J Clin Immunol. 2007;27:233–245. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources