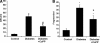Diabetes impairs the vascular recruitment of normal stem cells by oxidant damage, reversed by increases in pAMPK, heme oxygenase-1, and adiponectin - PubMed (original) (raw)
. 2009 Feb;27(2):399-407.
doi: 10.1634/stemcells.2008-0800.
Silvia Morbelli, Luca Vanella, Claudia Kusmic, Cecilia Marini, Michela Massollo, Carla Augeri, Mirko Corselli, Chiara Ghersi, Barbara Chiavarina, Luigi F Rodella, Antonio L'Abbate, George Drummond, Nader G Abraham, Francesco Frassoni
Affiliations
- PMID: 19038792
- PMCID: PMC2729677
- DOI: 10.1634/stemcells.2008-0800
Free PMC article
Diabetes impairs the vascular recruitment of normal stem cells by oxidant damage, reversed by increases in pAMPK, heme oxygenase-1, and adiponectin
Gianmario Sambuceti et al. Stem Cells. 2009 Feb.
Free PMC article
Abstract
Background: Atherosclerosis progression is accelerated in diabetes mellitus (DM) by either direct endothelial damage or reduced availability and function of endothelial progenitor cells (EPCs). Both alterations are related to increased oxidant damage.
Aim: We examined if DM specifically impairs vascular signaling, thereby reducing the recruitment of normal EPCs, and if increases in antioxidant levels by induction of heme oxygenase-1 (HO-1) can reverse this condition.
Methods: Control and diabetic rats were treated with the HO-1 inducer cobalt protoporphyrin (CoPP) once a week for 3 weeks. Eight weeks after the development of diabetes, EPCs harvested from the aorta of syngenic inbred normal rats and labeled with technetium-99m-exametazime were infused via the femoral vein to estimate their blood clearance and aortic recruitment. Circulating endothelial cells (CECs) and the aortic expression of thrombomodulin (TM), CD31, and endothelial nitric oxide synthase (eNOS) were used to measure endothelial damage.
Results: DM reduced blood clearance and aortic recruitment of EPCs. Both parameters were returned to control levels by CoPP treatment without affecting EPC kinetics in normal animals. These abnormalities of EPCs in DM were paralleled by reduced serum adiponectin levels, increased numbers of CECs, reduced endothelial expression of phosphorylated eNOS, and reduced levels of TM, CD31, and phosphorylated AMP-activated protein kinase (pAMPK). CoPP treatment restored all of these parameters to normal levels.
Conclusion: Type II DM and its related oxidant damage hamper the interaction between the vascular wall and normal EPCs by mechanisms that are, at least partially, reversed by the induction of HO-1 gene expression, adiponectin, and pAMPK levels.
Figures
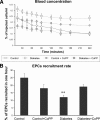
Figure 1
EPC kinetics in blood. (A): Time concentration curves of radioactivity in the blood are shown expressed as a percentage of the dose (and thus, of the labeled injected EPCs). Diabetes was associated with a prolonged persistence of EPCs in the blood, as seen by the higher values of radioactivity (and thus, of EPC concentration) at each time point. Induction of heme oxygenase-1 (HO-1) gene expression by CoPP treatment (gray symbols) selectively accelerated EPC clearance from the blood in diabetic animals (squares), without an effect on nondiabetic rats (circles). (B): Whole body clearance of EPCs. Diabetes was associated with a marked reduction in EPC recruitment in the body, expressed by a prolonged persistence of these cells in the blood (**p < .01), whereas CoPP treatment restored this variable to normal values. Abbreviations: CoPP, cobalt protoporphyrin; EPC, endothelial progenitor cell.
Figure 2
EPC recruitment rate in the aorta, lung, spleen, and liver. The recruitment rate was unaffected by diabetes and CoPP treatment in the liver and spleen. Reciprocal results are seen in the aorta and lung of diabetic animals. **p < .001, diabetic rats versus controls. Abbreviations: CoPP, cobalt protoporphyrin; EPC, endothelial progenitor cell.
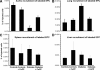
Figure 3
Molecular effects of CoPP treatment. (A): Enzyme-linked immunosorbent assay for the presence of serum oxidized proteins. CoPP restored normal values of oxidized proteins in the serum of diabetic rats; n = 5, *p < .01 versus control, **p < .01 versus diabetes. (B): Heme-oxygenase activity in the aorta, n = 4. CoPP treatment markedly increased Hemeoxygenase activity in diabetic rats, **p < .001 versus control and diabetic animals. (C): Adiponectin levels in the plasma of control rats, diabetic rats, and diabetic rats treated with CoPP. CoPP markedly increased serum concentration of this hormone in diabetes: n = 4. *p < .05 control versus Diabetic and control rats.
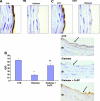
Figure 4
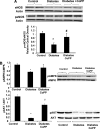
Immunohistochemical staining of TM (A) and CD31 (B) from control and diabetic animals. (C): Optical density analysis of TM staining in diabetic rats and its restoration by CoPP. *p < .05, control versus diabetic animals, mean ± standard error, n = 40. Abbreviations: CoPP, cobalt protoporphyrin; CTR, control; I, tunica intima; IOD, integrated optical density; M, tunica media; TM, thrombomodulin.
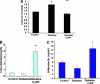
Figure 5
Reduction of endothelial damage by CoPP treatment. (A): The number of CECs increased significantly in STZ-induced diabetic rats (p < .05) relative to control rats. Administration of the heme oxygenase-1 inducer CoPP decreased the number of CECs, n = 6. *p < .05 versus controls; #p < .05 versus diabetic rats. (B): Similarly, endothelial cell membrane fragments in blood obtained from control rats were increased in diabetic rats and decreased after CoPP treatment, n = 5. *p < .001 versus controls; #p < .001 versus diabetic rats. Abbreviations: CEC, circulating endothelial cell; CoPP, cobalt protoporphyrin; STZ, streptozotocin.
Figure 6
Effect of CoPP treatment on endothelial gene expression. (A): Western blot and densitometry analysis of eNOS and peNOS from control, diabetic, and CoPP-treated diabetic rat aortas. Quantitative densitometry evaluation of eNOS and peNOS in the aorta was determined. Each bar represents the mean ± standard error of four experiments. *p < .001 for diabetic versus CoPP-treated diabetic rats. (B): Effect of diabetes and HO-1 expression on pAKT and total AKT in the aorta's proteins, and AMPK and pAMPK. Quantitative densitometry evaluations in aorta tissue homogenates of pAKT and pAMPK are expressed as a ratio to HO-2. *p < .01, diabetic rats versus control rats or CoPP-treated diabetic rats. Abbreviations: AKT, phosphatidylinositol 3-kinase; AMPK, AMP-activated protein kinase; CoPP, cobalt protoporphyrin; eNOS, endothelial nitric oxide synthase; HO, heme oxygenase; p, phosphorylated.
Similar articles
- Heme oxygenase-derived carbon monoxide restores vascular function in type 1 diabetes.
Rodella LF, Vanella L, Peterson SJ, Drummond G, Rezzani R, Falck JR, Abraham NG. Rodella LF, et al. Drug Metab Lett. 2008 Dec;2(4):290-300. doi: 10.2174/187231208786734058. Drug Metab Lett. 2008. PMID: 19356108 - Induction of Haemeoxygenase-1 Directly Improves Endothelial Function in Isolated Aortas from Obese Rats through the Ampk-Pi3k/Akt-Enos Pathway.
Han F, Guo Y, Xu L, Hou N, Han F, Sun X. Han F, et al. Cell Physiol Biochem. 2015;36(4):1480-90. doi: 10.1159/000430312. Cell Physiol Biochem. 2015. PMID: 26160485 - Long-term treatment with the apolipoprotein A1 mimetic peptide increases antioxidants and vascular repair in type I diabetic rats.
Peterson SJ, Husney D, Kruger AL, Olszanecki R, Ricci F, Rodella LF, Stacchiotti A, Rezzani R, McClung JA, Aronow WS, Ikehara S, Abraham NG. Peterson SJ, et al. J Pharmacol Exp Ther. 2007 Aug;322(2):514-20. doi: 10.1124/jpet.107.119479. Epub 2007 May 8. J Pharmacol Exp Ther. 2007. PMID: 17488882 - Induction of haemeoxygenase-1 improves FFA-induced endothelial dysfunction in rat aorta.
Han F, Hui Z, Zhang S, Hou N, Wang Y, Sun X. Han F, et al. Cell Physiol Biochem. 2015;35(3):1230-40. doi: 10.1159/000373946. Epub 2015 Feb 11. Cell Physiol Biochem. 2015. PMID: 25766533 - Nitric oxide: a key factor behind the dysfunctionality of endothelial progenitor cells in diabetes mellitus type-2.
Hamed S, Brenner B, Roguin A. Hamed S, et al. Cardiovasc Res. 2011 Jul 1;91(1):9-15. doi: 10.1093/cvr/cvq412. Epub 2010 Dec 24. Cardiovasc Res. 2011. PMID: 21186243 Review.
Cited by
- Vascular endothelium function among male carriers of BRCA 1&2 germline mutation.
Witberg G, Lev E, Ber Y, Tabachnik T, Sela S, Belo I, Leshem-Lev D, Margel D. Witberg G, et al. Oncotarget. 2019 Aug 20;10(49):5041-5051. doi: 10.18632/oncotarget.27118. eCollection 2019 Aug 20. Oncotarget. 2019. PMID: 31489114 Free PMC article. - Mononuclear cell therapy attenuates atherosclerosis in apoE KO mice.
Porto ML, Lima LC, Pereira TM, Nogueira BV, Tonini CL, Campagnaro BP, Meyrelles SS, Vasquez EC. Porto ML, et al. Lipids Health Dis. 2011 Sep 6;10:155. doi: 10.1186/1476-511X-10-155. Lipids Health Dis. 2011. PMID: 21896159 Free PMC article. - High fat diet enhances cardiac abnormalities in SHR rats: Protective role of heme oxygenase-adiponectin axis.
Cao J, Sodhi K, Puri N, Monu SR, Rezzani R, Abraham NG. Cao J, et al. Diabetol Metab Syndr. 2011 Dec 23;3(1):37. doi: 10.1186/1758-5996-3-37. Diabetol Metab Syndr. 2011. PMID: 22196253 Free PMC article. - (S)-1-α-naphthylmethyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline (CKD712), promotes wound closure by producing VEGF through HO-1 induction in human dermal fibroblasts and mouse skin.
Jang HJ, Tsoyi K, Kim YM, Park EJ, Park SW, Kim HJ, Lee JH, Chang KC. Jang HJ, et al. Br J Pharmacol. 2013 Mar;168(6):1485-96. doi: 10.1111/bph.12031. Br J Pharmacol. 2013. PMID: 23088309 Free PMC article. - Epoxyeicosatrienoic acid agonist rescues the metabolic syndrome phenotype of HO-2-null mice.
Sodhi K, Inoue K, Gotlinger KH, Canestraro M, Vanella L, Kim DH, Manthati VL, Koduru SR, Falck JR, Schwartzman ML, Abraham NG. Sodhi K, et al. J Pharmacol Exp Ther. 2009 Dec;331(3):906-16. doi: 10.1124/jpet.109.157545. Epub 2009 Aug 28. J Pharmacol Exp Ther. 2009. PMID: 19717790 Free PMC article.
References
- Bahia L, Aguiar LG, Villela N, et al. Relationship between adipokines, inflammation, and vascular reactivity in lean controls and obese subjects with metabolic syndrome. Clinics. 2006;61:433–440. - PubMed
- Kruger AL, Peterson S, Turkseven S, et al. D-4F induces heme oxygenase-1 and extracellular superoxide dismutase, decreases endothelial cell sloughing, and improves vascular reactivity in rat model of diabetes. Circulation. 2005;111:3126–3134. - PubMed
- Abraham NG, Kushida T, McClung J, et al. Heme oxygenase-1 attenuates glucose-mediated cell growth arrest and apoptosis in human microvessel endothelial cells. Circ Res. 2003;93:507–514. - PubMed
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. - PubMed
- Esper RJ, Vilarino JO, Machado RA, et al. Endothelial dysfunction in normal and abnormal glucose metabolism. Adv Cardiol. 2008;45:17–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL034300/HL/NHLBI NIH HHS/United States
- HL34300/HL/NHLBI NIH HHS/United States
- HL55601/HL/NHLBI NIH HHS/United States
- DK068134/DK/NIDDK NIH HHS/United States
- R01 DK056601/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical