Sleep-dependent activity of T cells and regulatory T cells - PubMed (original) (raw)
Sleep-dependent activity of T cells and regulatory T cells
T Bollinger et al. Clin Exp Immunol. 2009 Feb.
Abstract
A number of immunological functions are dependent on circadian rhythms and regular sleep. This has impact on the type and magnitude of immune responses following antigenic challenge, for example in vaccination. Little is known about the underlying mechanisms. One possibility may be the circadian and sleep-dependent modulation of CD4(+)CD25(-) T cell responses by CD4(+)CD25(+) natural regulatory T cells (nT(reg)). In a variety of studies, nT(reg) have been shown to regulate T cell responses negatively. Thus, we investigated the influence of sleep and circadian rhythm on the number and function of nT(reg) as well as on the function of CD4(+)CD25(-) T cells. Seven healthy young men were examined under defined conditions on two occasions, i.e. during sleep and sleep deprivation. Venous blood was drawn periodically; numbers of nT(reg), suppressive activity of nT(reg), interleukin-2 production and proliferation of CD4(+)CD25(-) T cells were explored in vitro. nT(reg) counts revealed a significant circadian rhythm with highest levels during the night (mean 95 nT(reg)/microl) and lowest levels during the day (mean 55 nT(reg)/microl). During normal sleep, the suppressive activity of nT(reg) was highest at 02.00 h and somewhat lower at 15.00 h. Surprisingly, almost no suppressive activity was present at 07.00 h. Deprivation of sleep abrogated this rhythm. CD4(+)CD25(-) T cell proliferation was dampened significantly by sleep deprivation. This is the first study in human cells to show that nT(reg) number and function follow a rhythm across the 24-h period. Furthermore, sleep deprivation severely disturbs the functional rhythm of nT(reg) and CD4(+)CD25(-) T cells.
Figures
Fig. 1
T cell and natural T regulatory (nTreg) cell purity and fluorescence activated cell sorter (FACS) analysis of CD4+CD25− T cell proliferation. (a) CD4+CD25− T cells (left panel) and CD4+CD25+ regulatory T cells (middle and right panel) were isolated from peripheral blood mononuclear cells (PBMC) applying magnetic affinity cell sorter (MACS®) technology. Purified T cells were stained with αCD4-monoclonal antibody (mAb) labelled with allophycocyanin and αCD25-mAb labelled with phycoerythrin or with αCD4-mAb labelled with fluorescein and αforkhead box P3 (FoxP3)-mAb labelled with allophycocyanin and were then analysed by flow cytometry. Mean purity of CD4+CD25− T cells was 96·8% ± 0·6% and mean purity of CD4+CD25+ nTreg was 79·4% ± 7·1%. (b) CD4+CD25−carboxyfluorescein diacetate (CFDA)+ T cells were cultured with nTreg (middle panel) or without nTreg (left panel) in the presence of irradiated adherent cells and αCD3-mAb. Proliferation of CD4+CD25−CFDA+ T cells was measured as the reduction in CFDA fluorescence. M2 represents the percentage of proliferated CD4+CD25− T cells. Control cultures were performed without αCD3-mAb (right panel). Data are from one representative experiment of 70.
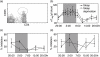
Fig. 2
Absolute counts of natural T regulatory (nTreg) and suppression of CD4+ T cell proliferation through nTreg. (a) To analyse the number of CD4+CD25+ regulatory T cells in peripheral blood we quantified the CD4+CD25high cells indicated by the circle. The percentage of forkhead box P3+ (FoxP3+) cells within the CD4+CD25high population did not differ over the 24-h period or between the sleep and sleep deprivation condition; 91·5% ± 1% of the CD4+CD25high cells were positive for FoxP3 (data not shown). (b) CD4+CD25+ regulatory T cells were counted in peripheral blood from healthy men during sleep (closed circles) or sleep deprivation (open circles). (c) CD4+CD25− T cells and nTreg were isolated from peripheral blood of healthy young men with sleep (closed circles) and without nocturnal sleep (open circles). CD4+CD25− T cells were stimulated polyclonally either in the presence or absence of nTreg. The inhibition of CD4+CD25− T cell proliferation by nTreg was calculated (for detailed information see Material and methods). (d) Peripheral blood mononuclear cells (PBMC) and PBMC depleted of nTreg were stimulated polyclonally and the inhibition of CD4+ T cell proliferation through the presence of nTreg was quantified in cells isolated from healthy young men with sleep (closed circles) or sleep deprivation (open circles). Shaded area indicates bedtime. Mean values ± standard error of the mean (n = 7).
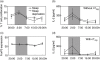
Fig. 3
Proliferation and interleukin (IL)-2 production of CD4+CD25− T cells in vitro and forkhead box P3 (FoxP3) expression in natural T regulatory (nTreg) cells. (a) CD4+CD25− T cells were isolated from peripheral blood of healthy young men with sleep (closed circles) or sleep deprivation (open circles), labelled with carboxyfluorescein diacetate (CFDA) and polyclonally stimulated. The percentage of proliferated T cell was measured through the reduction in CFDA fluorescence. (b, d) IL-2 was measured in the supernatants of polyclonally stimulated CD4+CD25− T cells in the absence (b) or presence (d) of nTreg. (c) FoxP3 expression levels in nTreg (CD4+CD25high) were measured as ‘Geo Mean’ applying flow cytometry. Cells were isolated from peripheral blood of healthy young men with sleep or sleep deprivation. Shaded area indicates bedtime. Mean values ± standard error of the mean (n = 7).
Similar articles
- CD4(+)CD25 (+) regulatory T cells in human lupus erythematosus.
Kuhn A, Beissert S, Krammer PH. Kuhn A, et al. Arch Dermatol Res. 2009 Jan;301(1):71-81. doi: 10.1007/s00403-008-0891-9. Epub 2008 Nov 5. Arch Dermatol Res. 2009. PMID: 18985367 Review. - Engagement of TLR2 reverses the suppressor function of conjunctiva CD4+CD25+ regulatory T cells and promotes herpes simplex virus epitope-specific CD4+CD25- effector T cell responses.
Dasgupta G, Chentoufi AA, You S, Falatoonzadeh P, Urbano LA, Akhtarmalik A, Nguyen K, Ablabutyan L, Nesburn AB, BenMohamed L. Dasgupta G, et al. Invest Ophthalmol Vis Sci. 2011 May 17;52(6):3321-33. doi: 10.1167/iovs.10-6522. Invest Ophthalmol Vis Sci. 2011. PMID: 21273544 Free PMC article. - TGF-beta1 modulates Foxp3 expression and regulatory activity in distinct CD4+ T cell subsets.
Pyzik M, Piccirillo CA. Pyzik M, et al. J Leukoc Biol. 2007 Aug;82(2):335-46. doi: 10.1189/jlb.1006644. Epub 2007 May 2. J Leukoc Biol. 2007. PMID: 17475784 - Oral tolerance induction with antigen conjugated to cholera toxin B subunit generates both Foxp3+CD25+ and Foxp3-CD25- CD4+ regulatory T cells.
Sun JB, Raghavan S, Sjöling A, Lundin S, Holmgren J. Sun JB, et al. J Immunol. 2006 Dec 1;177(11):7634-44. doi: 10.4049/jimmunol.177.11.7634. J Immunol. 2006. PMID: 17114433 - Type 1 T regulatory cells and their relationship with CD4+CD25+ T regulatory cells.
Roncarolo MG, Gregori S, Levings M. Roncarolo MG, et al. Novartis Found Symp. 2003;252:115-27; discussion 127-31, 203-10. Novartis Found Symp. 2003. PMID: 14609215 Review.
Cited by
- Anti-Citrullinated Cyclic Peptide Antibody and Functional Disability Are Associated With Poor Sleep Quality in Rheumatoid Arthritis.
Rajalingam S, Sakthiswary R, Hussein H. Rajalingam S, et al. Arch Rheumatol. 2016 Dec 8;32(1):15-20. doi: 10.5606/ArchRheumatol.2017.5960. eCollection 2017 Mar. Arch Rheumatol. 2016. PMID: 30375543 Free PMC article. - Sleep deprivation alters neutrophil functions and levels of Th1-related chemokines and CD4+ T cells in the blood.
Said EA, Al-Abri MA, Al-Saidi I, Al-Balushi MS, Al-Busaidi JZ, Al-Reesi I, Koh CY, Idris MA, Al-Jabri AA, Habbal O. Said EA, et al. Sleep Breath. 2019 Dec;23(4):1331-1339. doi: 10.1007/s11325-019-01851-1. Epub 2019 Apr 30. Sleep Breath. 2019. PMID: 31041780 - Association between sleep deficiency and cardiometabolic disease: implications for health disparities.
Rangaraj VR, Knutson KL. Rangaraj VR, et al. Sleep Med. 2016 Feb;18:19-35. doi: 10.1016/j.sleep.2015.02.535. Epub 2015 Mar 23. Sleep Med. 2016. PMID: 26431758 Free PMC article. Review. - Diurnal rhythms in blood cell populations and the effect of acute sleep deprivation in healthy young men.
Ackermann K, Revell VL, Lao O, Rombouts EJ, Skene DJ, Kayser M. Ackermann K, et al. Sleep. 2012 Jul 1;35(7):933-40. doi: 10.5665/sleep.1954. Sleep. 2012. PMID: 22754039 Free PMC article. - T Cells Plead for Rejuvenation and Amplification; With the Brain's Neurotransmitters and Neuropeptides We Can Make It Happen.
Levite M. Levite M. Front Immunol. 2021 Mar 22;12:617658. doi: 10.3389/fimmu.2021.617658. eCollection 2021. Front Immunol. 2021. PMID: 33868232 Free PMC article. Review.
References
- Boivin DB, James FO, Wu A, Cho-Park PF, Xiong H, Sun ZS. Circadian clock genes oscillate in human peripheral blood mononuclear cells. Blood. 2003;102:4143–5. - PubMed
- Bryant PA, Trinder J, Curtis N. Sick and tired: does sleep have a vital role in the immune system? Nat Rev Immunol. 2004;4:457–67. - PubMed
- Dimitrov S, Lange T, Tieken S, Fehm HL, Born J. Sleep associated regulation of T helper 1/T helper 2 cytokine balance in humans. Brain Behav Immun. 2004;18:341–8. - PubMed
- Dimitrov S, Lange T, Fehm HL, Born J. A regulatory role of prolactin, growth hormone, and corticosteroids for human T-cell production of cytokines. Brain Behav Immun. 2004;18:368–74. - PubMed
- Lange T, Dimitrov S, Fehm HL, Westermann J, Born J. Shift of monocyte function toward cellular immunity during sleep. Arch Intern Med. 2006;166:1695–700. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials