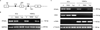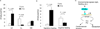Vascular PPARgamma controls circadian variation in blood pressure and heart rate through Bmal1 - PubMed (original) (raw)
Vascular PPARgamma controls circadian variation in blood pressure and heart rate through Bmal1
Ningning Wang et al. Cell Metab. 2008 Dec.
Abstract
Thiazolidinediones (TZDs) are PPARgamma activators that exhibit vasculoprotective properties. To determine the vascular function of PPARgamma, we analyzed Tie2Cre/flox and SM22Cre/flox mice. Unexpectedly, both knockout strains exhibited a significant reduction of circadian variations in blood pressure and heart rate in parallel with diminished variations in urinary norepinephrine/epinephrine excretion and impaired rhythmicity of the canonical clock genes, including Bmal1. PPARgamma expression in the aorta exhibited a robust rhythmicity with a more than 20-fold change during the light/dark cycle. Rosiglitazone treatment induced aortic expression of Bmal1 mRNA, and ChIP and promoter assays revealed that Bmal1 is a direct PPARgamma target gene. These studies have uncovered a role for vascular PPARgamma as a peripheral factor participating in regulation of cardiovascular rhythms.
Figures
Fig. 1
Validation of PPARγ deletion in the vascular cells. (A) Schematic illustration of primer design for detection of the PPARγ foxed allele (S1 and AS1) and PPARγ mRNA expression (S2 and AS2). (B) PCR analysis of the PPARγ floxed allele in ECs and VSMCs freshly isolated from PPARγf/f (f/f), SM22Cre/flox (SM22), Tie2Cre/flox (Tie2) mice using primers S1 and AS1. Amplification of GAPDH gene served as a loading control. C, RT-PCR analysis of PPARγ mRNA in ECs and VSMCs from the three strains of mice. CD31 and α-SMA are markers of EC and SMC, respectively. α-Tubulin was used as a loading control. Shown are representatives of 2~3 experiments.
Fig. 2
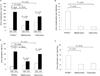
Altered diurnal rhythms in MAP and HR in SM22Cre/flox and Tie2Cre/flox mice. (A) Hourly recordings of MAP over 24 h. CT0, the beginning of a subjective circadian period (6:00am). (B) The average MAP during light and dark phases. (C) The maximal variations of MAP over 24 h. (D) Hourly recordings of HR over 24 h. (E) The average HR during light and dark phases. (C) The maximal variations of HR over 24 h. N=5–7 in each group. Data are mean ± SE.
Fig. 3
Altered diurnal rhythms in sympathetic activity in SM22Cre/flox and Tie2Cre/flox mice. (A) 12-h urine output of NE during light and dark phases. (B) Night-to-day ratios of urinary NE. (C) 12-h urine output of Epi. (D) Night-to-day ratios of urinary Epi. N=6–7 in each group. Data are mean ± SE.
Fig. 4
Real time RT-PCR of analysis of gene expressions in the aortae and livers of PPARγf/f, SM22Cre/flox and Tie2Cre/flox mice at 4-h intervals. X-axis represents circadian time (CT0, the beginning of subjective light cycle). (A) PPARγ expression in PPARγf/f mice; (B–I) Expressions of canonical clock genes in aortae and livers in the three mouse strains. N=5 in each time point. Shown are mean ± SE. *, P<0.05 and #, P<0.01 vs. PPARγf/f.
Fig. 5
PPARγ regulates Bmal1 gene transcription. PPARγf/f mice were treated with rosiglitazone (RGZ) at 320 mg/kg diet for 2 days. The thoracic aortae were harvested and subjected to real time RT-PCR (A) and ChIP assay (B). For luciferase assay, mouse SVEC4-10 cells (C) and mouse M1 cells (D) transiently transfected with constructs containing wild-type Bmal1 promoter or Bmal1 promoter with mutated PPRE, and cotransfected with expression vectors for PPARγ, RXRα, and β-galactosidase. In (E), the PPRE site is shown in green and mutated sequences are indicated by lower-case letters. Confluent cells were exposed for 24 h with vehicle or 2 µM RGZ. Luciferase activity was normalized by β-galactosidase activity. In (A), (C), and (D), N = 4–5 per group and data are mean ± SE. In (B), the two lanes in each group represent two separate animals.
Fig. 6
Effects of lightness/darkness and restricted feeding on the cyclic expression of vascular PPARγ. (A) PPARγ mRNA expression in the aortae of PPARγf/f mice kept under regular 12:12 h light/dark cycle (LD), constant lightness (LL), or constant darkness (DD) for 3 days. (B) PPARγ mRNA expression in the aortae of PPARγf/f mice kept under the regular LD cycle and fed only during the light or dark phase. All animals were killed at CT4 and CT16, and aortic PPARγ expression was determined by real time RT-PCR. N=5 in each group. Shown are mean ± SEM. (C) Proposed role of PPARγ in integrating the environmental signals, the clock, the cardiovascular function, and metabolism.
Similar articles
- Redundant function of REV-ERBalpha and beta and non-essential role for Bmal1 cycling in transcriptional regulation of intracellular circadian rhythms.
Liu AC, Tran HG, Zhang EE, Priest AA, Welsh DK, Kay SA. Liu AC, et al. PLoS Genet. 2008 Feb 29;4(2):e1000023. doi: 10.1371/journal.pgen.1000023. PLoS Genet. 2008. PMID: 18454201 Free PMC article. - Distinct functions of vascular endothelial and smooth muscle PPARgamma in regulation of blood pressure and vascular tone.
Wang N, Symons JD, Zhang H, Jia Z, Gonzalez FJ, Yang T. Wang N, et al. Toxicol Pathol. 2009 Jan;37(1):21-7. doi: 10.1177/0192623308328545. Epub 2008 Dec 15. Toxicol Pathol. 2009. PMID: 19075043 Free PMC article. - A Novel Bmal1 Mutant Mouse Reveals Essential Roles of the C-Terminal Domain on Circadian Rhythms.
Park N, Kim HD, Cheon S, Row H, Lee J, Han DH, Cho S, Kim K. Park N, et al. PLoS One. 2015 Sep 22;10(9):e0138661. doi: 10.1371/journal.pone.0138661. eCollection 2015. PLoS One. 2015. PMID: 26394143 Free PMC article. - Comment on "Differential rescue of light- and food-entrainable circadian rhythms".
Mistlberger RE, Yamazaki S, Pendergast JS, Landry GJ, Takumi T, Nakamura W. Mistlberger RE, et al. Science. 2008 Oct 31;322(5902):675; author reply 675. doi: 10.1126/science.1161387. Science. 2008. PMID: 18974333 Free PMC article. - [Synchronization and genetic redundancy in circadian clocks].
Dardente H. Dardente H. Med Sci (Paris). 2008 Mar;24(3):270-6. doi: 10.1051/medsci/2008243270. Med Sci (Paris). 2008. PMID: 18334175 Review. French.
Cited by
- Mechanism of the circadian clock in physiology.
Richards J, Gumz ML. Richards J, et al. Am J Physiol Regul Integr Comp Physiol. 2013 Jun 15;304(12):R1053-64. doi: 10.1152/ajpregu.00066.2013. Epub 2013 Apr 10. Am J Physiol Regul Integr Comp Physiol. 2013. PMID: 23576606 Free PMC article. Review. - Nocturia: The circadian voiding disorder.
Kim JW, Moon YT, Kim KD. Kim JW, et al. Investig Clin Urol. 2016 May;57(3):165-73. doi: 10.4111/icu.2016.57.3.165. Epub 2016 May 10. Investig Clin Urol. 2016. PMID: 27195315 Free PMC article. Review. - A single-cell transcriptomic atlas of exercise-induced anti-inflammatory and geroprotective effects across the body.
Sun S, Ma S, Cai Y, Wang S, Ren J, Yang Y, Ping J, Wang X, Zhang Y, Yan H, Li W, Esteban CR, Yu Y, Liu F, Izpisua Belmonte JC, Zhang W, Qu J, Liu GH. Sun S, et al. Innovation (Camb). 2023 Jan 5;4(1):100380. doi: 10.1016/j.xinn.2023.100380. eCollection 2023 Jan 30. Innovation (Camb). 2023. PMID: 36747595 Free PMC article. - Circadian Rhythm Effects on the Molecular Regulation of Physiological Systems.
Crislip GR, Johnston JG, Douma LG, Costello HM, Juffre A, Boyd K, Li W, Maugans CC, Gutierrez-Monreal M, Esser KA, Bryant AJ, Liu AC, Gumz ML. Crislip GR, et al. Compr Physiol. 2021 Dec 29;12(1):2769-2798. doi: 10.1002/cphy.c210011. Compr Physiol. 2021. PMID: 34964116 Free PMC article. Review. - Opposite Interplay Between the Canonical WNT/β-Catenin Pathway and PPAR Gamma: A Potential Therapeutic Target in Gliomas.
Vallée A, Lecarpentier Y, Guillevin R, Vallée JN. Vallée A, et al. Neurosci Bull. 2018 Jun;34(3):573-588. doi: 10.1007/s12264-018-0219-5. Epub 2018 Mar 26. Neurosci Bull. 2018. PMID: 29582250 Free PMC article. Review.
References
- Anan F, Masaki T, Fukunaga N, Teshima Y, Iwao T, Kaneda K, Umeno Y, Okada K, Wakasugi K, Yonemochi H, et al. Pioglitazone shift circadian rhythm of blood pressure from non-dipper to dipper type in type 2 diabetes mellitus. Eur J Clin Invest. 2007;37:709–714. - PubMed
- Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. - PubMed
- Berger JP, Akiyama TE, Meinke PT. PPARs: therapeutic targets for metabolic disease. Trends Pharmacol Sci. 2005;26:244–251. - PubMed
- Boucher P, Gotthardt M, Li WP, Anderson RG, Herz J. LRP: role in vascular wall integrity and protection from atherosclerosis. Science. 2003;300:329–332. - PubMed
- Buijs RM, Kalsbeek A. Hypothalamic integration of central and peripheral clocks. Nat Rev Neurosci. 2001;2:521–526. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases