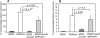The major surface-associated saccharides of Klebsiella pneumoniae contribute to host cell association - PubMed (original) (raw)
The major surface-associated saccharides of Klebsiella pneumoniae contribute to host cell association
Abigail Clements et al. PLoS One. 2008.
Abstract
Analysing the pathogenic mechanisms of a bacterium requires an understanding of the composition of the bacterial cell surface. The bacterial surface provides the first barrier against innate immune mechanisms as well as mediating attachment to cells/surfaces to resist clearance. We utilised a series of Klebsiella pneumoniae mutants in which the two major polysaccharide layers, capsule and lipopolysaccharide (LPS), were absent or truncated, to investigate the ability of these layers to protect against innate immune mechanisms and to associate with eukaryotic cells. The capsule alone was found to be essential for resistance to complement mediated killing while both capsule and LPS were involved in cell-association, albeit through different mechanisms. The capsule impeded cell-association while the LPS saccharides increased cell-association in a non-specific manner. The electrohydrodynamic characteristics of the strains suggested the differing interaction of each bacterial strain with eukaryotic cells could be partly explained by the charge density displayed by the outermost polysaccharide layer. This highlights the importance of considering not only specific adhesin:ligand interactions commonly studied in adherence assays but also the initial non-specific interactions governed largely by the electrostatic interaction forces.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
Figure 1. Characterisation of the surface layers of K. pneumoniae mutant strains.
A) The capsule was visualised by Maneval's stain and quantified by uronic acid assay. UD = undetectable. B) LPS preparations were separated by SDS-PAGE on a 15% polyacrylamide gel and visualised by modified silver staining. C) Fluorescent activated cell sorting using mAb C13 specific for the O1-Ag of LPS. Only single cell populations (determined from cells stained with secondary antibody alone) were included in fluorescence analsyis. D) OMP preparations from mid-log cultures separated by 12.5% SDS-PAGE and visualised by Coomassie Brilliant Blue staining. Lanes are (1) B5055, (2) B5055nm, (3) B5055nm_waaF_ and (4) B5055nm_waaF_+pBR_waaF_.
Figure 2. Association of K. pneumoniae mutant strains to mammalian cells.
Bacteria were cultured to mid-log growth phase, washed, and added to confluent monolayers of RAW264.7 macrophage-like cells (A) or A549 epithelial cells (B). After 2 hrs, non-adherent bacteria were removed by repeated washing, cells were treated with Triton X-100 and cell lysates were plated for CFU enumeration. The number of cell associated bacteria are presented as the percentage of the initial inoculum. The data are the mean and standard deviation (SD) of 6 wells/strain and the assay was repeated at least three times.
Figure 3. K. pneumoniae CPS and LPS saccharides affect the electrophoretic mobility of the bacteria.
The electrophoretic mobility of B5055, B5055nm, B5055nm_waaF_ and B5055nm_waaF_ (pBR_waaF_) were compared in different ionic strength potassium nitrate buffers at the natural pH of the buffer (pH 5.5–6). Shown are the mean and SD of 60 trajectories for each strain, under each condition. The assay was repeated on different days with fresh cultures.
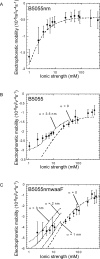
Figure 4. Theoretical description of the experimental electrophoretic mobilities of K. pneumoniae strains.
The electrophoretic mobility (filled circles) are graphed for A) B5055nm, B) B5055 and C) B5055nm_waaF K. pneumoniae_ strains expressed as a function of ionic strength. The calculations were performed either by using a homogeneous description for the spatial distribution of the soft-permeable polymer (α = 0, dash lines) or using a heterogeneous distribution (α>0, dotted lines) to reproduce the experimental data over the whole range of ionic strength considered. The different parameters obtained from the best fitting procedure are indicated in Table 1.
References
- Vogel U, Hammerschmidt S, Frosch M. Sialic acids of both the capsule and the sialylated lipooligosaccharide of Neisseria meningitis serogroup B are prerequisites for virulence of meningococci in the infant rat. Med Microbiol Immunol. 1996;185:81–87. - PubMed
- Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–745. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources