Brain-derived neurotrophic factor functions as a metabotrophin to mediate the effects of exercise on cognition - PubMed (original) (raw)
Brain-derived neurotrophic factor functions as a metabotrophin to mediate the effects of exercise on cognition
Fernando Gomez-Pinilla et al. Eur J Neurosci. 2008 Dec.
Abstract
Brain-derived neurotrophic factor (BDNF) has been shown to mediate the effects of exercise on synaptic plasticity and cognitive function, in a process in which energy metabolism probably plays an important role. The purpose of the present study was to examine the influence of exercise on rat hippocampal expression of molecules involved in the regulation of energy management and cognitive function, and to determine the role of BDNF in these events. One week of voluntary exercise that enhanced learning and memory performance elevated the expression of molecular systems involved in the metabolism of energy [AMP-activated protein kinase (AMPK), ubiquitous mitochondrial creatine kinase (uMtCK) and uncoupling protein 2] and molecules that work at the interface of energy and synaptic plasticity [BDNF, insulin-like growth factor I (IGF-I) and ghrelin]. The levels of BDNF mRNA were associated with the mRNA levels of AMPK, uMtCK, IGF-I and ghrelin. Inhibiting the action of BDNF during exercise abolished an exercise-mediated enhancement in spatial learning and increased the expression of all of the molecular systems studied. BDNF blocking also disrupted the association between learning speed and levels of AMPK, uMtCK, ghrelin and IGF-I mRNAs. These findings suggest that the effects of exercise on synaptic plasticity and cognitive function involve elements of energy metabolism, and that BDNF seems to work at the interface between the two processes as a metabotrophin.
Figures
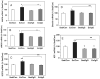
Fig. 1
Effects of voluntary exercise on the mRNA levels of AMPK (A), uMtCK (B), UCP-2 (C), ghrelin (D) and IGF-I (E) in the hippocampus. Exercise (Ex/Con) elevated the expression of these metabolism-related molecules relative to sedentary animals (Sed/Con). The injection of the BDNF inhibitor TrkB-IgG into the hippocampus abolished these increases in exercise rats (Ex/IgG). Levels of AMPK, uMtCK and UCP-2 are expressed as a percent of sedentary controls (Sed/Con). Each value represents the mean ± SEM (two-way anova, *P < 0.05, **P < 0.01). Sed/IgG, sedentary with TrkB-IgG injection.
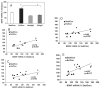
Fig. 2
Association between BDNF and metabolic molecular systems. (A) Exercise (Ex/Con) elevated the levels of BDNF mRNA relative to sedentary animals (Sed/Con) and this effect was counteracted by the hippocampal injection of the BDNF function blocker TrkB-IgG (Ex/IgG). The injection of TrkB-IgG also reduced BDNF mRNA levels in sedentary animals (Sed/IgG). Analysis of correlation showed an association between BDNF mRNA levels and mRNA levels of AMPK (B), uMtCK (C), ghrelin (D) and IGF-I (E) for sedentary and exercised animals. The injection of TrkB-IgG into the hippocampus of sedentary and exercised rats abolished the correlation (data not shown). Each value represents the mean ± SEM (two-way anova, *P < 0.05).
Fig. 3
(A) Exercise (Ex/Con) decreased the latency to locate the platform on days 3–5 in the MWM as compared with sedentary controls (Sed/Con). Blocking BDNF action with TrkB-IgG abolished the exercise-induced enhancement of learning acquisition (Ex/TrkB-IgG) but did not alter the escape latencies in the sedentary condition (Sed/IgG). (B) These effects were reflected in changes in the slope of the learning curve for the exercise condition. TrkB-IgG showed a decreasing trend in the sedentary group. A correlation analysis showed an association between learning speed and mRNA levels for AMPK (C), uMtCK (D), IGF-I (E) and ghrelin (F) for the total pool of exercise and sedentary rats. The association between learning speed and levels of mRNAs was disrupted by the injection of the BDNF blocker TrkB-IgG (data not shown). Each value represents the mean ± SEM (two-way anova, *P < 0.05, #P < 0.01).
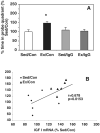
Fig. 4
(A) The probe trial showed a preference of exercised rats (Ex/Con) for the quadrant in which they had to swim for 60 s in the pool in which they received their training but with the escape platform removed. Blocking BDNF action prevented the exercise-induced preference for the target quadrant (Ex/IgG). Blocking the action of BDNF was selective for exercise as it did not affect the learning acquisition or recall abilities of sedentary animals (Sed/IgG). Each value represents the mean ± SEM (anova, *P < 0.05). (B) We performed a correlation analysis to evaluate a possible association between energy molecules and memory retention. We found a positive correlation between IGF-I mRNA levels and the memory retention ability (r = 0.68, P = 0.01) in the sedentary control (Sed/Con) and Ex/Con rats for the total pool of exercise and sedentary rats. The association between retention and levels of mRNAs was disrupted by the injection of the BDNF blocker TrkB-IgG (data not shown).
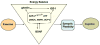
Fig. 5
Proposed mechanism by which exercise enhances cognitive function by engaging aspects of cellular energy metabolism. There is a crucial association between metabolic energy and synaptic plasticity, in which BDNF plays a crucial role. The effects of exercise on hippocampal BDNF would activate several molecular systems involved in the metabolism of energy, thereby modulating the capacity of the synapse to process information relevant to cognitive function. In particular, molecular systems, such as uMtCK, AMPK and UCP-2, may work at the interface between energy and synaptic plasticity. IGF-I, ghrelin and energy-related molecules can interact with BDNF to modulate synaptic plasticity and cognitive function. Therefore, BDNF appears to be a central integrator for the effects of exercise on synaptic markers and energy metabolic processes to affect cognitive function.
Similar articles
- Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function.
Ding Q, Vaynman S, Akhavan M, Ying Z, Gomez-Pinilla F. Ding Q, et al. Neuroscience. 2006 Jul 7;140(3):823-33. doi: 10.1016/j.neuroscience.2006.02.084. Epub 2006 May 2. Neuroscience. 2006. PMID: 16650607 - Coupling energy metabolism with a mechanism to support brain-derived neurotrophic factor-mediated synaptic plasticity.
Vaynman S, Ying Z, Wu A, Gomez-Pinilla F. Vaynman S, et al. Neuroscience. 2006;139(4):1221-34. doi: 10.1016/j.neuroscience.2006.01.062. Epub 2006 Mar 31. Neuroscience. 2006. PMID: 16580138 - Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition.
Vaynman S, Ying Z, Gomez-Pinilla F. Vaynman S, et al. Eur J Neurosci. 2004 Nov;20(10):2580-90. doi: 10.1111/j.1460-9568.2004.03720.x. Eur J Neurosci. 2004. PMID: 15548201 - Role of exercise-induced brain-derived neurotrophic factor production in the regulation of energy homeostasis in mammals.
Pedersen BK, Pedersen M, Krabbe KS, Bruunsgaard H, Matthews VB, Febbraio MA. Pedersen BK, et al. Exp Physiol. 2009 Dec;94(12):1153-60. doi: 10.1113/expphysiol.2009.048561. Epub 2009 Sep 11. Exp Physiol. 2009. PMID: 19748969 Review. - Exercise: a behavioral intervention to enhance brain health and plasticity.
Cotman CW, Berchtold NC. Cotman CW, et al. Trends Neurosci. 2002 Jun;25(6):295-301. doi: 10.1016/s0166-2236(02)02143-4. Trends Neurosci. 2002. PMID: 12086747 Review.
Cited by
- Physical Activity and Brain Health.
Di Liegro CM, Schiera G, Proia P, Di Liegro I. Di Liegro CM, et al. Genes (Basel). 2019 Sep 17;10(9):720. doi: 10.3390/genes10090720. Genes (Basel). 2019. PMID: 31533339 Free PMC article. Review. - Physical exercise augmented cognitive behaviour therapy for older adults with generalised anxiety disorder (PEXACOG): study protocol for a randomized controlled trial.
Stavestrand SH, Sirevåg K, Nordhus IH, Sjøbø T, Endal TB, Nordahl HM, Specht K, Hammar Å, Halmøy A, Martinsen EW, Andersson E, Hjelmervik H, Mohlman J, Thayer JF, Hovland A. Stavestrand SH, et al. Trials. 2019 Mar 18;20(1):174. doi: 10.1186/s13063-019-3268-9. Trials. 2019. PMID: 30885256 Free PMC article. - Increased adult hippocampal neurogenesis is not necessary for wheel running to abolish conditioned place preference for cocaine in mice.
Mustroph ML, Merritt JR, Holloway AL, Pinardo H, Miller DS, Kilby CN, Bucko P, Wyer A, Rhodes JS. Mustroph ML, et al. Eur J Neurosci. 2015 Jan;41(2):216-26. doi: 10.1111/ejn.12782. Epub 2014 Nov 13. Eur J Neurosci. 2015. PMID: 25393660 Free PMC article. - Physical exercise as an epigenetic modulator of brain plasticity and cognition.
Fernandes J, Arida RM, Gomez-Pinilla F. Fernandes J, et al. Neurosci Biobehav Rev. 2017 Sep;80:443-456. doi: 10.1016/j.neubiorev.2017.06.012. Epub 2017 Jun 27. Neurosci Biobehav Rev. 2017. PMID: 28666827 Free PMC article. Review.
References
- Alsina B, Vu T, Cohen-Cory S. Visualizing synapse formation in arborizing optic axons in vivo: dynamics and modulation by BDNF. Nat Neurosci. 2001;4:1093–1101. - PubMed
- Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31:571–591. - PubMed
- Anlar B, Sullivan KA, Feldman EL. Insulin-like growth factor-I and central nervous system development. Horm Metab Res. 1999;31:120–125. - PubMed
- Araujo DM, Lapchak PA, Collier B, Chabot JG, Quirion R. Insulin-like growth factor-1 (somatomedin-C) receptors in the rat brain: distribution and interaction with the hippocampal cholinergic system. Brain Res. 1989;484:130–138. - PubMed
- Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, Suda M, Koh T, Natsui K, Toyooka S, Shirakami G, Usui T, Shimatsu A, Doi K, Hosoda H, Kojima M, Kangawa K, Nakao K. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab. 2001;86:4753–4758. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources