ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear - PubMed (original) (raw)
. 2008 Dec 2;105(48):18770-5.
doi: 10.1073/pnas.0800793105. Epub 2008 Dec 1.
Victor H Hernandez, Giulia Crispino, Anke Seydel, Saida Ortolano, Stephen D Roper, Nicoletta Kessaris, William Richardson, Gesa Rickheit, Mikhail A Filippov, Hannah Monyer, Fabio Mammano
Affiliations
- PMID: 19047635
- PMCID: PMC2596208
- DOI: 10.1073/pnas.0800793105
ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear
Fabio Anselmi et al. Proc Natl Acad Sci U S A. 2008.
Abstract
Extracellular ATP controls various signaling systems including propagation of intercellular Ca(2+) signals (ICS). Connexin hemichannels, P2x7 receptors (P2x7Rs), pannexin channels, anion channels, vesicles, and transporters are putative conduits for ATP release, but their involvement in ICS remains controversial. We investigated ICS in cochlear organotypic cultures, in which ATP acts as an IP(3)-generating agonist and evokes Ca(2+) responses that have been linked to noise-induced hearing loss and development of hair cell-afferent synapses. Focal delivery of ATP or photostimulation with caged IP(3) elicited Ca(2+) responses that spread radially to several orders of unstimulated cells. Furthermore, we recorded robust Ca(2+) signals from an ATP biosensor apposed to supporting cells outside the photostimulated area in WT cultures. ICS propagated normally in cultures lacking either P2x7R or pannexin-1 (Px1), as well as in WT cultures exposed to blockers of anion channels. By contrast, Ca(2+) responses failed to propagate in cultures with defective expression of connexin 26 (Cx26) or Cx30. A companion paper demonstrates that, if expression of either Cx26 or Cx30 is blocked, expression of the other is markedly down-regulated in the outer sulcus. Lanthanum, a connexin hemichannel blocker that does not affect gap junction (GJ) channels when applied extracellularly, limited the propagation of Ca(2+) responses to cells adjacent to the photostimulated area. Our results demonstrate that these connexins play a dual crucial role in inner ear Ca(2+) signaling: as hemichannels, they promote ATP release, sustaining long-range ICS propagation; as GJ channels, they allow diffusion of Ca(2+)-mobilizing second messengers across coupled cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
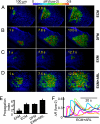
Fig. 1.
Effect of divalent ions and ectonucleotidases on ICS evoked by ATP puffs in the organ of Corti. (A-D) Representative experiments showing pseudocolor images of Ca2+ signals (measured as fura-2 emission ratio changes; Δ_R_) propagating through cochlear supporting and epithelial cells of P3-P6 mouse organotypic cultures following focal delivery of an ATP puff (50 ms, 4 μM) from a glass micropipette with a tip opening of ≈1.5 μm. EXM, extracellular medium containing 2 mM Ca2+; ECM, medium containing endolymphatic Ca2+ concentration (20 μM); DFM, medium devoid of divalent ions; EXM+ARL, ectonucleotidase inhibitor ARL67156 (100 μM) dissolved in EXM. Time of image capture measured from the end of ATP delivery is indicated on each frame. (E) Pooled data showing ICS propagation index for conditions in A-D; data are mean ± SEM for independently repeated experiments on at least five cultures from different animals; **P < 0.01, Student t test. (F) Representative whole-cell averages of fura-2 Δ_R_ versus time for randomly selected cells in a culture bathed in ECM and stimulated with 100 μM ARL67156 (empty bar at bottom marks timing of drug delivery).
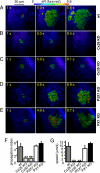
Fig. 2.
Ca2+signals evoked by 100 ms of focal photostimulation with caged IP3 fail to propagate in connexin-deficient cochlear cultures but propagate normally in Px1 KO and P2x7 KO cultures. (A-E) Ca2+ signals in cochlear supporting and epithelial cells from P3-P6 mouse organotypic cultures bathed in ECM and co-loaded with the AM esters of fura red and caged IP3. (A) Control (WT culture); (B) Cx26 KO culture; (C) Cx30 KO culture; (D) P2x7 KO culture; (E) Px1 KO culture. (F) Pooled data showing propagation range in WT controls and in cultures lacking Cx26, Cx30, P2x7, or Px1. (G) Speed of propagating ICS for data in F. Data in F and G are mean ± SEM for independently repeated experiments in tissues from at least three different animals.
Fig. 3.
Ca2+responses evoked in ATP biosensors by 100 ms of focal photostimulation of cochlear supporting cells with caged IP3. (A) Example of a pipette-held biosensor loaded with fluo-4 and apposed to a Hensen's cell in a culture loaded with caged IP3 AM and viewed with bright-field differential interference contrast optics. (B) The same field under conventional epifluorescence illumination. (C) Representative biosensor whole-cell pixel averages of percent fluo-4 fluorescence change (Δ_F_) relative to pre-stimulus fluorescence emission intensity (_F_0) measured before delivery of the photostimulation (arrow, stimulus onset), versus time for similar independently repeated experiments performed in tissues from three different animals.
Fig. 4.
Calcein efflux from supporting cells of the organ of Corti. Left, time course of percent fluorescence emission change (Δ_F_) relative to initial fluorescence intensity (_F_0) in cultures preloaded with calcein AM. Right, efflux rate from data at left. Values shown as mean ± SEM for independently repeated experiments on cultures from at least four different animals.
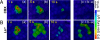
Fig. 5.
Effects of CBX and La3+ on the spread of Ca2+ signals evoked by 100 ms of photostimulation with caged IP3 in the organ of Corti. Ca2+ responses of cultures incubated with 100 μM CBX (A) or 100 μM La3+ (B). The fourth image in each sequence is the pixel-by-pixel difference (i.e., c = b − a) of the first two images. Results are representative of similar independently repeated experiments performed in tissues from at least three different animals.
Similar articles
- ATP-mediated cell-cell signaling in the organ of Corti: the role of connexin channels.
Majumder P, Crispino G, Rodriguez L, Ciubotaru CD, Anselmi F, Piazza V, Bortolozzi M, Mammano F. Majumder P, et al. Purinergic Signal. 2010 Jun;6(2):167-87. doi: 10.1007/s11302-010-9192-9. Epub 2010 Jun 17. Purinergic Signal. 2010. PMID: 20806010 Free PMC article. - Coordinated control of connexin 26 and connexin 30 at the regulatory and functional level in the inner ear.
Ortolano S, Di Pasquale G, Crispino G, Anselmi F, Mammano F, Chiorini JA. Ortolano S, et al. Proc Natl Acad Sci U S A. 2008 Dec 2;105(48):18776-81. doi: 10.1073/pnas.0800831105. Epub 2008 Dec 1. Proc Natl Acad Sci U S A. 2008. PMID: 19047647 Free PMC article. - Photoliberating inositol-1,4,5-trisphosphate triggers ATP release that is blocked by the connexin mimetic peptide gap 26.
Braet K, Vandamme W, Martin PE, Evans WH, Leybaert L. Braet K, et al. Cell Calcium. 2003 Jan;33(1):37-48. doi: 10.1016/s0143-4160(02)00180-x. Cell Calcium. 2003. PMID: 12526886 - Connexin and pannexin hemichannels in inflammatory responses of glia and neurons.
Bennett MV, Garré JM, Orellana JA, Bukauskas FF, Nedergaard M, Sáez JC. Bennett MV, et al. Brain Res. 2012 Dec 3;1487:3-15. doi: 10.1016/j.brainres.2012.08.042. Epub 2012 Sep 10. Brain Res. 2012. PMID: 22975435 Free PMC article. Review. - Connexins and gap junctions in the inner ear--it's not just about K⁺ recycling.
Jagger DJ, Forge A. Jagger DJ, et al. Cell Tissue Res. 2015 Jun;360(3):633-44. doi: 10.1007/s00441-014-2029-z. Epub 2014 Nov 9. Cell Tissue Res. 2015. PMID: 25381570 Free PMC article. Review.
Cited by
- An Ala/Glu difference in E1 of Cx26 and Cx30 contributes to their differential anionic permeabilities.
Kraujaliene L, Kraujalis T, Snipas M, Verselis VK. Kraujaliene L, et al. J Gen Physiol. 2024 Nov 4;156(11):e202413600. doi: 10.1085/jgp.202413600. Epub 2024 Sep 20. J Gen Physiol. 2024. PMID: 39302317 Free PMC article. - A pore locus in the E1 domain differentially regulates Cx26 and Cx30 hemichannel function.
Sanchez HA, Kraujaliene L, Verselis VK. Sanchez HA, et al. J Gen Physiol. 2024 Nov 4;156(11):e202313502. doi: 10.1085/jgp.202313502. Epub 2024 Sep 20. J Gen Physiol. 2024. PMID: 39302316 Free PMC article. - Innexin expression and localization in the Drosophila antenna indicate gap junction or hemichannel involvement in antennal chemosensory sensilla.
Prelic S, Keesey IW, Lavista-Llanos S, Hansson BS, Wicher D. Prelic S, et al. Cell Tissue Res. 2024 Oct;398(1):35-62. doi: 10.1007/s00441-024-03909-3. Epub 2024 Aug 23. Cell Tissue Res. 2024. PMID: 39174822 Free PMC article. - Deletion of murine astrocytic vesicular nucleotide transporter increases anxiety and depressive-like behavior and attenuates motivation for reward.
Huang Q, Lee HH, Volpe B, Zhang Q, Xue C, Liu BC, Abuhasan YR, Li L, Yang JS, Egholm J, Gutierrez-Vazquez C, Li A, Lee A, Tang S, Wong CW, Liu T, Huang Y, Ramos RL, Stout RF, El Ouaamari A, Quintana FJ, Lowell BB, Kahn CR, Pothos EN, Cai W. Huang Q, et al. Mol Psychiatry. 2024 Aug 9. doi: 10.1038/s41380-024-02692-5. Online ahead of print. Mol Psychiatry. 2024. PMID: 39122778 - Calcium Regulation of Connexin Hemichannels.
Bayraktar E, Lopez-Pigozzi D, Bortolozzi M. Bayraktar E, et al. Int J Mol Sci. 2024 Jun 15;25(12):6594. doi: 10.3390/ijms25126594. Int J Mol Sci. 2024. PMID: 38928300 Free PMC article. Review.
References
- Krstic RV. Human Microscopic Anatomy. Berlin: Springer-Verlag; 1997.
- Rio C, Dikkes P, Liberman MC, Corfas G. Glial fibrillary acidic protein expression and promoter activity in the inner ear of developing and adult mice. J Comp Neurol. 2002;442:156–162. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- G0501173/MRC_/Medical Research Council/United Kingdom
- R01 DC007630/DC/NIDCD NIH HHS/United States
- G9708005/MRC_/Medical Research Council/United Kingdom
- G0800575/MRC_/Medical Research Council/United Kingdom
- R55 DC007630/DC/NIDCD NIH HHS/United States
- DC007630/DC/NIDCD NIH HHS/United States
- GGP05131/TI_/Telethon/Italy
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous