High-programmed death-1 levels on hepatitis C virus-specific T cells during acute infection are associated with viral persistence and require preservation of cognate antigen during chronic infection - PubMed (original) (raw)
Comparative Study
. 2008 Dec 15;181(12):8215-25.
doi: 10.4049/jimmunol.181.12.8215.
Stuart C Ray, Jacquie Astemborski, Jordana Levine, Lin Liu, Kimberly A Dowd, Shalyn Clute, Changyu Wang, Alan Korman, Alessandro Sette, John Sidney, Drew M Pardoll, Andrea L Cox
Affiliations
- PMID: 19050238
- PMCID: PMC2773824
- DOI: 10.4049/jimmunol.181.12.8215
Comparative Study
High-programmed death-1 levels on hepatitis C virus-specific T cells during acute infection are associated with viral persistence and require preservation of cognate antigen during chronic infection
Alleluiah Rutebemberwa et al. J Immunol. 2008.
Abstract
Hepatitis C virus (HCV) is an important human pathogen that represents a model for chronic infection given that the majority of infected individuals fail to clear the infection despite generation of virus-specific T cell responses during the period of acute infection. Although viral sequence evolution at targeted MHC class I-restricted epitopes represents one mechanism for immune escape in HCV, many targeted epitopes remain intact under circumstances of viral persistence. To explore alternative mechanisms of HCV immune evasion, we analyzed patterns of expression of a major inhibitory receptor on T cells, programmed death-1 (PD-1), from the time of initial infection and correlated these with HCV RNA levels, outcome of infection, and sequence escape within the targeted epitope. We show that the level of PD-1 expression in early HCV infection is significantly higher on HCV-specific T cells from subjects who progress to chronic HCV infection than from those who clear infection. This correlation is independent of HCV RNA levels, compatible with the notion that high PD-1 expression on HCV-specific CD8 T cells during acute infection inhibits viral clearance. Viral escape during persistent infection is associated with reduction in PD-1 levels on the surface of HCV-specific T cells, supporting the necessity of ongoing antigenic stimulation of T cells for maintenance of PD-1 expression. These results support the idea that PD-1 expression on T cells specific for nonescaped epitopes contributes to viral persistence and suggest that PD-1 blockade may alter the outcome of HCV infection.
Figures
Figure 1. PD-1 staining patterns for two representative subjects
PD-1 expression on HCV-specific T cells was essentially uniformly positive and generally unimodal. Therefore, the mean levels of PD-1 expression (mean fluorescence intensity, MFI) on the entire multimers positive population of T cells rather than percentage positivity was used as an unbiased estimate of PD-1 expression. The numbers in the top boxes indicate the percentage of isotype control (top row) or PD-1 positive (bottom row) CD8 positive HCV-specific T cells.
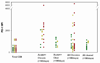
Figure 2. PD-1 expression on HCV-specific T cells is higher than on influenza-specific T cells and the general CD8 T cell population regardless of outcome of infection or the phase of infection
The MFI of PD-1 on the T cell surface was compared between HCV specific T cells from subjects with either outcome of infection, control specific T cells and the general CD8 population in the first 180 days of HCV infection (HCV+ acute to chronic or cleared, 2a) and at time points following 180 days of HCV infection (HCV+ chronic or cleared, 2b). The level of PD-1 expression is also higher in acute infection on HCV specific T cells from subjects who remain persistently infected than those who clear HCV infection. Each data point represents the MFI of the PD-1 level on a specific tetramer positive population within an individual.
Figure 3. PD-1 expression on HCV specific T cells stratified by HCV RNA level during early and late infection
HCV RNA levels at the time the T cells were acquired are indicated by colored symbols. Red: >100,000 copies/mL Yellow: 600–100,000 copies/mL Green: <600 copies/mL. Acute infection was defined as time points less than 180 days from initial viremia. PD-1 levels are highly variable in chronic infection. Some HCV-specific T cells from subjects with chronic infection expressed low PD-1 levels in the setting of high circulating HCV RNA levels (Red dots at low PD-1 MFI values).
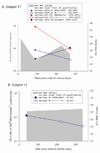
Figure 4. PD-1 upregulation requires maintenance of intact antigen and restoration of intact antigen following escape is associated with an increase in PD-1 levels
Viral sequence was assessed at initial infection and at multiple subsequent time points at which T cells specific for known antigens were detectable, including those surrounding the time at which the PD-1 levels were found to be low relative to initial PD-1 levels. Levels of PD-1 on T cells specific for epitopes that either underwent substitutions that impaired T cell recognition or remained constant are shown. Time points at which the substituted epitopes were present are indicated with open circles and points at which the initial viral sequence was present are indicated with closed circles. A. Subject 17 recognized three epitopes: 880TL10 and B35-135 underwent substitution in the first six months of infection and 140G remained intact. PD-1 levels decline three and ten fold following substitution of 880TL10 and B35-135, respectively. T cells recognizing 140G demonstrated a three fold increase in PD-1 expression. B. Subject 13 had one HCV specific T cell response that underwent substitution with an associated drop of nearly three fold in PD-1 expression. C. In Subject 175, HCV levels transiently fell to below the limit of quantitation with a decline in PD-1 levels followed by reemergence of high viral titers and an increase in PD-1 levels on T cells specific for the epitope. D. Subject 30. Sequence escape at epitope C63B is associated with a five-fold decline in PD-1 levels. C63B reverted back to the original sequence in association with a six-fold increase in PD-1 expression on the cognate T cells. A2-61 does not undergo substitution and the level of PD-1 expression varies less than two fold. PD-1 levels are maintained constant or increase over time in the absence of viral sequence variation. (E–H) Levels of PD-1 on T cells specific for epitopes that remained constant are shown for four different HCV infected subjects who recognized at least one T cell epitope for which multimers were available. For all four subjects, PD-1 expression on HCV-specific T cells decreases less than two-fold or increases over time.
Figure 4. PD-1 upregulation requires maintenance of intact antigen and restoration of intact antigen following escape is associated with an increase in PD-1 levels
Viral sequence was assessed at initial infection and at multiple subsequent time points at which T cells specific for known antigens were detectable, including those surrounding the time at which the PD-1 levels were found to be low relative to initial PD-1 levels. Levels of PD-1 on T cells specific for epitopes that either underwent substitutions that impaired T cell recognition or remained constant are shown. Time points at which the substituted epitopes were present are indicated with open circles and points at which the initial viral sequence was present are indicated with closed circles. A. Subject 17 recognized three epitopes: 880TL10 and B35-135 underwent substitution in the first six months of infection and 140G remained intact. PD-1 levels decline three and ten fold following substitution of 880TL10 and B35-135, respectively. T cells recognizing 140G demonstrated a three fold increase in PD-1 expression. B. Subject 13 had one HCV specific T cell response that underwent substitution with an associated drop of nearly three fold in PD-1 expression. C. In Subject 175, HCV levels transiently fell to below the limit of quantitation with a decline in PD-1 levels followed by reemergence of high viral titers and an increase in PD-1 levels on T cells specific for the epitope. D. Subject 30. Sequence escape at epitope C63B is associated with a five-fold decline in PD-1 levels. C63B reverted back to the original sequence in association with a six-fold increase in PD-1 expression on the cognate T cells. A2-61 does not undergo substitution and the level of PD-1 expression varies less than two fold. PD-1 levels are maintained constant or increase over time in the absence of viral sequence variation. (E–H) Levels of PD-1 on T cells specific for epitopes that remained constant are shown for four different HCV infected subjects who recognized at least one T cell epitope for which multimers were available. For all four subjects, PD-1 expression on HCV-specific T cells decreases less than two-fold or increases over time.
Figure 4. PD-1 upregulation requires maintenance of intact antigen and restoration of intact antigen following escape is associated with an increase in PD-1 levels
Viral sequence was assessed at initial infection and at multiple subsequent time points at which T cells specific for known antigens were detectable, including those surrounding the time at which the PD-1 levels were found to be low relative to initial PD-1 levels. Levels of PD-1 on T cells specific for epitopes that either underwent substitutions that impaired T cell recognition or remained constant are shown. Time points at which the substituted epitopes were present are indicated with open circles and points at which the initial viral sequence was present are indicated with closed circles. A. Subject 17 recognized three epitopes: 880TL10 and B35-135 underwent substitution in the first six months of infection and 140G remained intact. PD-1 levels decline three and ten fold following substitution of 880TL10 and B35-135, respectively. T cells recognizing 140G demonstrated a three fold increase in PD-1 expression. B. Subject 13 had one HCV specific T cell response that underwent substitution with an associated drop of nearly three fold in PD-1 expression. C. In Subject 175, HCV levels transiently fell to below the limit of quantitation with a decline in PD-1 levels followed by reemergence of high viral titers and an increase in PD-1 levels on T cells specific for the epitope. D. Subject 30. Sequence escape at epitope C63B is associated with a five-fold decline in PD-1 levels. C63B reverted back to the original sequence in association with a six-fold increase in PD-1 expression on the cognate T cells. A2-61 does not undergo substitution and the level of PD-1 expression varies less than two fold. PD-1 levels are maintained constant or increase over time in the absence of viral sequence variation. (E–H) Levels of PD-1 on T cells specific for epitopes that remained constant are shown for four different HCV infected subjects who recognized at least one T cell epitope for which multimers were available. For all four subjects, PD-1 expression on HCV-specific T cells decreases less than two-fold or increases over time.
Figure 4. PD-1 upregulation requires maintenance of intact antigen and restoration of intact antigen following escape is associated with an increase in PD-1 levels
Viral sequence was assessed at initial infection and at multiple subsequent time points at which T cells specific for known antigens were detectable, including those surrounding the time at which the PD-1 levels were found to be low relative to initial PD-1 levels. Levels of PD-1 on T cells specific for epitopes that either underwent substitutions that impaired T cell recognition or remained constant are shown. Time points at which the substituted epitopes were present are indicated with open circles and points at which the initial viral sequence was present are indicated with closed circles. A. Subject 17 recognized three epitopes: 880TL10 and B35-135 underwent substitution in the first six months of infection and 140G remained intact. PD-1 levels decline three and ten fold following substitution of 880TL10 and B35-135, respectively. T cells recognizing 140G demonstrated a three fold increase in PD-1 expression. B. Subject 13 had one HCV specific T cell response that underwent substitution with an associated drop of nearly three fold in PD-1 expression. C. In Subject 175, HCV levels transiently fell to below the limit of quantitation with a decline in PD-1 levels followed by reemergence of high viral titers and an increase in PD-1 levels on T cells specific for the epitope. D. Subject 30. Sequence escape at epitope C63B is associated with a five-fold decline in PD-1 levels. C63B reverted back to the original sequence in association with a six-fold increase in PD-1 expression on the cognate T cells. A2-61 does not undergo substitution and the level of PD-1 expression varies less than two fold. PD-1 levels are maintained constant or increase over time in the absence of viral sequence variation. (E–H) Levels of PD-1 on T cells specific for epitopes that remained constant are shown for four different HCV infected subjects who recognized at least one T cell epitope for which multimers were available. For all four subjects, PD-1 expression on HCV-specific T cells decreases less than two-fold or increases over time.
Figure 5. Amino acid substitutions in T cell epitopes reduce recognition by cognate T cells, validating them as escape mutations
PBMC were cultivated in the presence of the peptide present at initial infection (closed circle) or at the point where PD-1 levels decreased (open circle) in Subject 17 (A) or Subject 30 (B). After 20 days of stimulation with the peptide present in initial infection (left panels) or the variant peptide (right panels), cells were tested for recognition of both peptides in serial dilutions in an IFN-γ Elispot assay. The number of spot forming colonies (SFC) per 30,000 cells is shown at each peptide dilution. In both cases, the variant peptide was less well recognized than the initial peptide by T cells expanded with the initial peptide (left panels). Stimulation with either of the variant peptides also resulted in reduced recognition of either the initial or variant peptides (right panels) and in reduced expansion of T cells as measured by T cell numbers (data not shown) compared to stimulation with the initial peptide. These substitutions were thus deemed escape mutations.
Similar articles
- Costimulatory molecule programmed death-1 in the cytotoxic response during chronic hepatitis C.
Larrubia JR, Benito-Martínez S, Miquel J, Calvino M, Sanz-de-Villalobos E, Parra-Cid T. Larrubia JR, et al. World J Gastroenterol. 2009 Nov 7;15(41):5129-40. doi: 10.3748/wjg.15.5129. World J Gastroenterol. 2009. PMID: 19891011 Free PMC article. Review. - Functional restoration of HCV-specific CD8 T cells by PD-1 blockade is defined by PD-1 expression and compartmentalization.
Nakamoto N, Kaplan DE, Coleclough J, Li Y, Valiga ME, Kaminski M, Shaked A, Olthoff K, Gostick E, Price DA, Freeman GJ, Wherry EJ, Chang KM. Nakamoto N, et al. Gastroenterology. 2008 Jun;134(7):1927-37, 1937.e1-2. doi: 10.1053/j.gastro.2008.02.033. Epub 2008 Feb 17. Gastroenterology. 2008. PMID: 18549878 Free PMC article. - Tim-3 expression on PD-1+ HCV-specific human CTLs is associated with viral persistence, and its blockade restores hepatocyte-directed in vitro cytotoxicity.
McMahan RH, Golden-Mason L, Nishimura MI, McMahon BJ, Kemper M, Allen TM, Gretch DR, Rosen HR. McMahan RH, et al. J Clin Invest. 2010 Dec;120(12):4546-57. doi: 10.1172/JCI43127. Epub 2010 Nov 15. J Clin Invest. 2010. PMID: 21084749 Free PMC article. - Upregulation of PD-1 expression on circulating and intrahepatic hepatitis C virus-specific CD8+ T cells associated with reversible immune dysfunction.
Golden-Mason L, Palmer B, Klarquist J, Mengshol JA, Castelblanco N, Rosen HR. Golden-Mason L, et al. J Virol. 2007 Sep;81(17):9249-58. doi: 10.1128/JVI.00409-07. Epub 2007 Jun 13. J Virol. 2007. PMID: 17567698 Free PMC article. - Mutational escape from CD8+ T cell immunity: HCV evolution, from chimpanzees to man.
Bowen DG, Walker CM. Bowen DG, et al. J Exp Med. 2005 Jun 6;201(11):1709-14. doi: 10.1084/jem.20050808. J Exp Med. 2005. PMID: 15939787 Free PMC article. Review.
Cited by
- Costimulatory molecule programmed death-1 in the cytotoxic response during chronic hepatitis C.
Larrubia JR, Benito-Martínez S, Miquel J, Calvino M, Sanz-de-Villalobos E, Parra-Cid T. Larrubia JR, et al. World J Gastroenterol. 2009 Nov 7;15(41):5129-40. doi: 10.3748/wjg.15.5129. World J Gastroenterol. 2009. PMID: 19891011 Free PMC article. Review. - Adaptive immunity to hepatitis C virus.
Zeisel MB, Fafi-Kremer S, Robinet E, Habersetzer F, Baumert TF, Stoll-Keller F. Zeisel MB, et al. Viruses. 2009 Sep;1(2):276-97. doi: 10.3390/v1020276. Epub 2009 Sep 8. Viruses. 2009. PMID: 21994550 Free PMC article. - Programmed death-1/programmed death-L1 signaling pathway and its blockade in hepatitis C virus immunotherapy.
Salem ML, El-Badawy A. Salem ML, et al. World J Hepatol. 2015 Oct 18;7(23):2449-58. doi: 10.4254/wjh.v7.i23.2449. World J Hepatol. 2015. PMID: 26483866 Free PMC article. Review. - CD8+ Tregs in lupus, autoimmunity, and beyond.
Dinesh RK, Skaggs BJ, La Cava A, Hahn BH, Singh RP. Dinesh RK, et al. Autoimmun Rev. 2010 Jun;9(8):560-8. doi: 10.1016/j.autrev.2010.03.006. Epub 2010 Apr 10. Autoimmun Rev. 2010. PMID: 20385256 Free PMC article. Review. - Adaptive immunity to the hepatitis C virus.
Walker CM. Walker CM. Adv Virus Res. 2010;78:43-86. doi: 10.1016/B978-0-12-385032-4.00002-1. Adv Virus Res. 2010. PMID: 21040831 Free PMC article. Review.
References
- World Health Organization. Hepatitis C: global prevalence. Weekly Epidemiological Record. 1997:341–348. - PubMed
- Alter MJ. Epidemiology of hepatitis C in the West. Semin Liver Dis. 1995;15:5–14. - PubMed
- Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR. 1998;47:1–39. (No. RR-19) - PubMed
- Villano SA, Vlahov D, Nelson KE, Cohn S, Thomas DL. Persistence of viremia and the importance of long-term follow-up after acute hepatitis C infection. Hepatology. 1999;29:908–914. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U19 AI040035-130003/AI/NIAID NIH HHS/United States
- U19 AI040035/AI/NIAID NIH HHS/United States
- K08 DA016535-05/DA/NIDA NIH HHS/United States
- N01AI40035/AI/NIAID NIH HHS/United States
- T32 AI007247/AI/NIAID NIH HHS/United States
- R01 DA024565-02/DA/NIDA NIH HHS/United States
- K08 DA11880/DA/NIDA NIH HHS/United States
- U19 AI040035-139001/AI/NIAID NIH HHS/United States
- R01 DA024565/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials