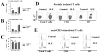Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys - PubMed (original) (raw)
Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys
José C Crispín et al. J Immunol. 2008.
Abstract
Double negative (DN) T cells are expanded in patients with systemic lupus erythematosus (SLE) and stimulate autoantibody production as efficiently as CD4(+) T cells. In this study, we demonstrate that DN T cells from patients with SLE produce significant amounts of IL-17 and IFN-gamma, and expand when stimulated in vitro with an anti-CD3 Ab in the presence of accessory cells. Furthermore, IL-17(+) and DN T cells are found in kidney biopsies of patients with lupus nephritis. Our findings establish that DN T cells produce the inflammatory cytokines IL-17 and IFN-gamma, and suggest that they contribute to the pathogenesis of kidney damage in patients with SLE.
Figures
FIGURE 1
An abnormally high fraction of T cells from patients with SLE produce IL-17. IL-17 expression was quantified in T cells, either immediately after isolation (A), or after 5-day culture in the absence (B) or presence (C) of plate-bound anti-CD3. At day 6, intracellular staining was performed. Cumulative data from 14 patients with SLE (black bars) and 12 healthy controls (white bars) is presented. *p<0.05. D. Freshly isolated T cells were treated with brefeldin A and stimulated for 3 hours with PMA and ionomycin before staining for flow cytometry. Representative dot plots (IL-17-Alexa fluor 647 vs. FSC; Total T cells, CD4+ T cells, DN T cells) from a healthy control and a patient with SLE are depicted. The gate was set according to an isotype control antibody. E. T cells stimulated during 5 days with plate-bound CD3 in the presence of accessory cells were harvested and stained for flow cytometry analysis. Representative histograms of a control and a SLE patient are shown. Dotted line represents isotype control staining.
FIGURE 2
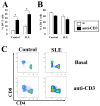
Double negative cells expand significantly following anti-CD3 stimulation. T cells were incubated for 5 days with autologous accessory cells. TCRαβ+ cells were gated and single positive (CD4+CD8−; CD4−CD8+) and DN (CD4−CD8−) cells were quantified in 8 patients with SLE and 7 healthy controls. The percentage of DN (A) and CD4 (B) T cells from normal subjects and SLE patients in the absence (white bars) and presence (black bars) of plate-bound anti-CD3. Representative samples from a control and a patient are presented (C). * p < 0.05.
FIGURE 3
Double negative cells produce IFN-γ and TNF-α. T cells were stimulated with plate-bound anti-CD3 in the presence of accessory cells. At day 6, cytokine expression was determined by intracellular cytokine staining. Representative histograms comparing IL-2 (A), IFN-γ (B), and TNF-α (C) expression in DN (red lines) and CD4+ (blue lines) cells from control individuals (upper panels) and patients with SLE (lower panels). Shaded area represents isotype control. The black bar indicates the area considered positive. On the right panels, cumulative data from 6 patients and 6 controls is presented as mean + SEM. White bars represent CD4+ T cells; black bars DN T cells. * p < 0.05.
FIGURE 4
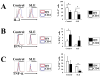
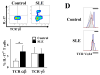
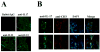
TCR γδ+ T cells and NKT cells are not involved in the increased IL-17 production of SLE patients. IL-17 expression was analyzed in recently isolated T cells. Live lymphocytes were gated according to FSC and SSC characteristics. Next, T cells were identified by the CD3 marker. TCR γδ and intracellular IL-17 expression was analyzed in the CD4− CD8− cell subset (DN T cells). Representative plots of a control and a patient (A), as well as cumulative data from 6 SLE patients and 4 controls (B) are shown (*_p_=0.05). C. Kidney sections from four SLE lupus nephritis patients were stained with mouse anti-human TCR γδ-FITC and rabbit anti-human IL-17 followed by goat anti-rabbit Texas Red. Slides were scanned in a confocal microscope and analyzed for the presence of TCR γδ+ cells. A representative field is shown. D. Expression of the NKT cell-associated TCR Vα24 was investigated in CD4+ and DN T cells from patients and controls (_n_=8). Although the frequency of the positive population varied among individuals, it remained confined to the CD4+ cell subset. Representative histograms of a control and a patient are shown. Shaded area indicates isotype control; blue line CD4+ T cells; red line DN T cells. The area considered positive is indicated by the black bar. E. Kidney sections from patients with lupus nephritis (_n_=4) were stained with mouse anti-human TCR Vα24 and rabbit anti-human CD3 followed by goat anti-mouse-Texas Red and goat anti-rabbit-FITC. Few NKT cells were observed. Shown is a representative field in which two NKT cells are marked with white circles.
FIGURE 5
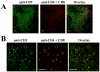
IL-17 and IL-23 are detected in affected kidneys from patients with SLE. A. Frozen sections were incubated with either rabbit Ig (control; upper left panel), rabbit anti-human IL-17 (upper right panel), or rabbit anti-human IL-23 (lower panels). Goat anti-rabbit labeled with Alexa fluor 488 was used as secondary antibody. B. Sections were stained with rabbit anti-human IL-17 and mouse anti-human CD3. Goat-anti rabbit-Alexa fluor 488 and goat anti-mouse-Texas Red were used as secondary antibodies and DAPI was used for nuclear staining. White bar represents 5 μm.
FIGURE 6
DN cells are interspersed with CD4+ and CD8+ T cells in cellular infiltrates of SLE kidney biopsies. Frozen sections were stained with rabbit anti-human CD3 and goat anti-human CD4 mixed with goat anti-human CD8. Goat-anti rabbit-Alexa fluor 488 and goat anti-mouse-Texas Red were used as secondary antibodies and DAPI was used for nuclear staining (A). In B, arrows point to large (~15 μm) CD4/8+ CD3− cells, most probably macrophages. Arrowheads indicate conventional single positive cells in which CD3 and either CD4 or CD8 colocalized. DN T cells are circled. White bar measures 50 μm.
Similar articles
- Differential expression of CD30 on CD3 T lymphocytes in patients with systemic lupus erythematosus.
Cabrera CM, Urra JM, Carreño A, Zamorano J. Cabrera CM, et al. Scand J Immunol. 2013 Sep;78(3):306-12. doi: 10.1111/sji.12088. Scand J Immunol. 2013. PMID: 23790231 - Human TCR-alpha beta+ CD4- CD8- T cells can derive from CD8+ T cells and display an inflammatory effector phenotype.
Crispín JC, Tsokos GC. Crispín JC, et al. J Immunol. 2009 Oct 1;183(7):4675-81. doi: 10.4049/jimmunol.0901533. Epub 2009 Sep 4. J Immunol. 2009. PMID: 19734235 Free PMC article. - A subset of CD4+ T cells expressing early activation antigen CD69 in murine lupus: possible abnormal regulatory role for cytokine imbalance.
Ishikawa S, Akakura S, Abe M, Terashima K, Chijiiwa K, Nishimura H, Hirose S, Shirai T. Ishikawa S, et al. J Immunol. 1998 Aug 1;161(3):1267-73. J Immunol. 1998. PMID: 9686587 - Effector T-cell subsets in systemic lupus erythematosus: update focusing on Th17 cells.
Shin MS, Lee N, Kang I. Shin MS, et al. Curr Opin Rheumatol. 2011 Sep;23(5):444-8. doi: 10.1097/BOR.0b013e328349a255. Curr Opin Rheumatol. 2011. PMID: 21720245 Free PMC article. Review. - IL-17 in systemic lupus erythematosus.
Crispín JC, Tsokos GC. Crispín JC, et al. J Biomed Biotechnol. 2010;2010:943254. doi: 10.1155/2010/943254. Epub 2010 Apr 6. J Biomed Biotechnol. 2010. PMID: 20379379 Free PMC article. Review.
Cited by
- Associations between T-cell traits and narcolepsy type 1: new insights from a Mendelian randomization study.
Chen S, Lv T, Li Z, Pan G, Chen Y, Zhao X, Zhang L. Chen S, et al. Front Neurol. 2024 Oct 31;15:1444753. doi: 10.3389/fneur.2024.1444753. eCollection 2024. Front Neurol. 2024. PMID: 39544989 Free PMC article. - Therapeutic potential of natural coumarins in autoimmune diseases with underlying mechanisms.
Li Y, Wang GQ, Li YB. Li Y, et al. Front Immunol. 2024 Oct 31;15:1432846. doi: 10.3389/fimmu.2024.1432846. eCollection 2024. Front Immunol. 2024. PMID: 39544933 Free PMC article. Review. - Enhanced ROS Production and Mitochondrial Metabolic Shifts in CD4+ T Cells of an Autoimmune Uveitis Model.
Söth R, Hoffmann ALC, Deeg CA. Söth R, et al. Int J Mol Sci. 2024 Oct 26;25(21):11513. doi: 10.3390/ijms252111513. Int J Mol Sci. 2024. PMID: 39519064 Free PMC article. - Lymphocytes Change Their Phenotype and Function in Systemic Lupus Erythematosus and Lupus Nephritis.
Moysidou E, Christodoulou M, Lioulios G, Stai S, Karamitsos T, Dimitroulas T, Fylaktou A, Stangou M. Moysidou E, et al. Int J Mol Sci. 2024 Oct 10;25(20):10905. doi: 10.3390/ijms252010905. Int J Mol Sci. 2024. PMID: 39456692 Free PMC article. Review. - DOES THE PRESENCE OF CHRONIC LYMPHOCYTIC THYROIDITIS AFFECT DIAGNOSTIC VALUE OF FINE NEEDLE ASPIRATION BIOPSY IN BETHESDA CATEGORY III NODULES?
Pedük Ş, Koçer B. Pedük Ş, et al. Acta Clin Croat. 2023 Nov;62(3):464-472. doi: 10.20471/acc.2023.62.03.8. Acta Clin Croat. 2023. PMID: 39310687 Free PMC article.
References
- Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med. 2008;358:929–939. - PubMed
- Adalid-Peralta L, Mathian A, Tran T, Delbos L, Durand-Gasselin I, Berrebi D, Peuchmaur M, Couderc J, Emilie D, Koutouzov S. Leukocytes and the kidney contribute to interstitial inflammation in lupus nephritis. Kidney Int. 2008;73:172–180. - PubMed
- Abe S, Amagasaki Y, Iyori S, Konishi K, Kato E, Sakaguchi H. Significance of tubulointerstitial lesions in biopsy specimens of glomerulonephritic patients. Am J Nephrol. 1989;9:30–37. - PubMed
- Austin HA, III, Boumpas DT, Vaughan EM, Balow JE. Predicting renal outcomes in severe lupus nephritis: contributions of clinical and histologic data. Kidney Int. 1994;45:544–550. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI042269-12A1/AI/NIAID NIH HHS/United States
- R01 AI049954-07A1/AI/NIAID NIH HHS/United States
- K23 AR055672/AR/NIAMS NIH HHS/United States
- R01 AI049954/AI/NIAID NIH HHS/United States
- K23 AR055672-01A1/AR/NIAMS NIH HHS/United States
- R01 AI073542-03/AI/NIAID NIH HHS/United States
- R01 AI42269/AI/NIAID NIH HHS/United States
- R01 AI042269/AI/NIAID NIH HHS/United States
- R01 AI49954/AI/NIAID NIH HHS/United States
- R01 AI073542-01/AI/NIAID NIH HHS/United States
- R01 AI073542/AI/NIAID NIH HHS/United States
- R01 AI073542-02/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials