Hair follicle stem cell-specific PPARgamma deletion causes scarring alopecia - PubMed (original) (raw)
Hair follicle stem cell-specific PPARgamma deletion causes scarring alopecia
Pratima Karnik et al. J Invest Dermatol. 2009 May.
Abstract
Primary cicatricial or scarring alopecias (CA) are a group of inflammatory hair disorders of unknown pathogenesis characterized by the permanent destruction of the hair follicle. The current treatment options are ineffective in controlling disease progression largely because the molecular basis for CA is not understood. Microarray analysis of the lymphocytic CA, Lichen planopilaris (LPP), compared to normal scalp biopsies identified decreased expression of genes required for lipid metabolism and peroxisome biogenesis. Immunohistochemical analysis showed progressive loss of peroxisomes, proinflammatory lipid accumulation, and infiltration of inflammatory cells followed by destruction of the pilosebaceous unit. The expression of peroxisome proliferator-activated receptor (PPAR) gamma, a transcription factor that regulates these processes, is significantly decreased in LPP. Specific agonists of PPARgamma are effective in inducing peroxisomal and lipid metabolic gene expression in human keratinocytes. Finally, targeted deletion of PPARgamma in follicular stem cells in mice causes a skin and hair phenotype that emulates scarring alopecia. These studies suggest that PPARgamma is crucial for healthy pilosebaceous units and it is the loss of this function that triggers the pathogenesis of LPP. We propose that PPARgamma-targeted therapy may represent a new strategy in the treatment of these disorders.
Conflict of interest statement
CONFLICT OF INTEREST
The authors state no conflict of interest.
Figures
Figure 1. Histology of normal and LPP scalp tissue
(a) Structure of the human hair follicle and the sebaceous glands (the pilosebaceous unit). The hair follicle stem cells are located in the hair follicle bulge region (b) between the arrector pili muscle (APM) and the sebaceous gland (SG). Other parts of the hair follicle depicted are the outer root sheath (ORS), inner root sheath (IRS), bulb (Bb), dermal papilla (DP), and matrix (M). (b) In scarring alopecias, inflammation occurs in the permanent portion of the hair follicle (infundibular region; striped area). Hematoxylin and eosin (H&E) staining of scalp biopsy sections (region of sebaceous glands) of normal at (c; × 10) and (d) Well-formed pilosebaceous units (× 20). LPP tissue sections (infundibular region) at (e; × 10) and (f; × 20) have very few hair follicles, atrophied sebaceous glands, and dense lymphocytic infiltrate around many hair follicles. Scale bar = 25 µm.
Figure 2. Peroxisome deficiency in LPP
Staining of peroxisomes with the anti-PMP-70 primary antibody for the peroxisomal membrane protein and Alexa 488-labeled secondary antibodies. In normal scalp tissue (normal), a characteristic “punctate” pattern of peroxisome staining is seen specifically in the sebaceous glands (SG) and in the ORS and IRS surrounding the hair shaft (HS). In unaffected tissue (uninvolved), peroxisome staining is lost in the ORS and IRS cells surrounding the hair shaft but not in sebaceous glands. Double staining of the unaffected tissue sections for peroxisomes and nuclei with (DAPI) shows that the ORS and IRS cells around the hair shaft are intact but the cells have lost peroxisomes (uninvolved, lower panel). Affected LPP tissue (LPP) shows a complete lack of staining for peroxisomes in the sebaceous glands and in the ORS and IRS cells surrounding the hair shaft. In LPP tissue, peroxisome staining is not seen in both the sebaceous gland and the hair follicle. Double staining the tissue for peroxisomes and nuclei reveals the presence of an intact sebaceous gland and hair follicle in LPP tissue (right, lower panel), suggesting the specific loss of peroxisomes. Scale bar = 25 µm.
Figure 3. Surface plot analysis of peroxisomes in normal scalp tissue sections and in LPP
High-magnification (× 40) confocal microscopy of (a) normal scalp sections, stained for peroxisomes with the anti-PMP-70 primary antibody, yielded a punctate pattern indicating the presence of peroxisomal membranes and intact peroxisomes. In LPP (b), no PMP-70-positive particles were visible indicating the complete absence of intact peroxisomes (c). Normal scalp section minus primary antibody control showed an absence of peroxisomal staining. Three-dimensional graphs (d–f) of the intensities of pixels in the peroxisomal images were determined by surface plot analysis with the NIH software Image J. The intensity of peroxisomal staining within a defined region (white rectangular areas highlighted in a–c) is interpreted as height for the plot and gives a comparative semiquantitative measure of the number of peroxisomes within the region in (d) normal, (e) LPP tissue and (f) normal minus primary antibody control. Scale bar = 25 µm.
Figure 4. Altered PPARγ gene expression in LPP and its effect on PEX and COX2 genes
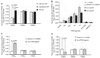
(a) PPAR gene expression in normal and LPP tissue. Total RNA was isolated from normal control (N = 10), unaffected LPP (N = 10), and affected LPP scalp tissue (N = 10) and PPARγ, PPARα, and PPARδ gene expression was measured by real-time PCR. PPARγ expression was significantly decreased in unaffected and affected LPP tissue. However, the expression of PPARα and PPARδ remained unchanged in LPP compared to control samples. (b) PPARγ modulation affects PEX16 gene expression in vitro. HaCaT keratinocytes were treated with vehicle alone (0.1% DMSO) or with specific agonists (1 and 5 µ
m
in 0.1% DMSO) of PPARγ-Ciglitazone (Cig), Rosiglitazone (Rosi), Pioglitazone (Pio), and Troglitazone (Tro), PPARα-WY-14363 and PPARδ–GW50516. After 48 hours, PEX16 gene expression was measured by real-time PCR. PPARγ agonists significantly induced the expression of PEX16 gene; however, PPARγ and PPARδ agonists had minimal effect. *represents P value ≤0.005. (c) PPARγ modulation affects PEX3 gene expression in outer root sheath (ORS) keratinocytes in vitro. In ORS cells, PPARγ agonist (Pioglitazone) significantly induced PEX3 gene expression compared to vehicle alone (0.1% DMSO). In contrast, PPARα–(WY-14363) and PPARδ (GW50516) agonists had minimal effect, suggesting that PPARγ regulates peroxisome biogenesis in the pilosebaceous units. (d) PPARγ agonist (Pio) significantly inhibited COX2 gene expression in ORS whereas PPARα–(WY-14363) and PPARδ (GW50516) agonists had minimal effect, suggesting that PPARγ negatively regulates COX2 expression.
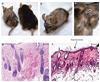
Figure 5. Targeted disruption of PPARγ in stem cells of the bulge resulted in scarring alopecia
(a) and (b) _PPARγ_fl/fl/Cre mice (females, 3 months) with _PPARγ_fl/fl littermate (male). Hair loss occurs in a random patchy and progressive manner. The mice display severe scratching behavior and are smaller than their normal littermates. (c) A close-up of the _PPARγ_fl/fl/Cre mice shows a region with absence of follicular markings (indicated by arrow) and erythema suggesting the presence of inflammation. The _PPARγ_fl/fl littermates have normal skin and hair phenotype. Histology of skin of _PPARγ_fl/fl and _PPARγ_fl/fl/Cre mice by Hematoxylin and eosin (H&E) staining of (d) _PPARγ_fl/fl mice shows normal hair follicles and sebaceous glands. (e) H&E staining of a _PPARγ_fl/fl/Cre mouse shows hyperkeratosis and follicular plugging (indicated by arrow). The sebaceous glands appeared normal in the early stage disease (2–3 months). Scale bar = 50 µm.
Figure 6. _PPARγ_fl/fl/Cre mice show histopathological features of scarring alopecia
H&E staining of the skin of PPARyfl/fl/Cre mice showed (a) dystrophic hair follicles, (b) follicular plugging, (c) dystrophic sebaceous glands, Scale bar = 20 µm, (d) interstitial, and (e) perifollicular inflammation. Scale bar = 50 µm.
Figure 7. _PPARγ_fl/fl/Cre mouse skin shows scarring
H&E staining showed (a) normal hair follicles in wild-type mouse skin (b) Dystrophic hair follicle with sebaceous gland atrophy in the skin of PPARγfl/fl/Cre mice; (c–e) follicular scarring, in which fibrous connective tissue strands run perpendicular to the epidermis from remnants of dystrophic hair follicles in _PPARγ_fl/fl/Cre mouse >4 months of age (H&E; × 40). Scale bar = 50 µm.
Figure 8. Proposed model for the pathogenesis of primary cicatricial alopecia
In normal pilosebaceous units, PPARγ binds to PPAR response elements (PPREs) on target genes and maintains lipid homeostasis by regulating peroxisome biogenesis and lipid metabolism. PPARγ also modulates the inflammatory response by regulating the expression of cytokine genes. In primary CA, PPARγ deficiency (either because of environmental or genetic factors) causes loss of peroxisome biogenesis, deregulates lipid metabolism, and produces proinflammatory lipids that trigger an inflammatory response that in turn causes tissue damage and permanent hair loss in CA.
Comment in
- Scarring alopecia and the PPAR-gamma connection.
Harries MJ, Paus R. Harries MJ, et al. J Invest Dermatol. 2009 May;129(5):1066-70. doi: 10.1038/jid.2008.425. J Invest Dermatol. 2009. PMID: 19369934
Similar articles
- Scarring alopecia and the PPAR-gamma connection.
Harries MJ, Paus R. Harries MJ, et al. J Invest Dermatol. 2009 May;129(5):1066-70. doi: 10.1038/jid.2008.425. J Invest Dermatol. 2009. PMID: 19369934 - PPAR-γ signalling as a key mediator of human hair follicle physiology and pathology.
Ramot Y, Bertolini M, Boboljova M, Uchida Y, Paus R. Ramot Y, et al. Exp Dermatol. 2020 Mar;29(3):312-321. doi: 10.1111/exd.14062. Epub 2019 Dec 17. Exp Dermatol. 2020. PMID: 31769892 Review. - Delineating immune-mediated mechanisms underlying hair follicle destruction in the mouse mutant defolliculated.
Ruge F, Glavini A, Gallimore AM, Richards HE, Thomas CP, O'Donnell VB, Philpott MP, Porter RM. Ruge F, et al. J Invest Dermatol. 2011 Mar;131(3):572-9. doi: 10.1038/jid.2010.379. Epub 2010 Dec 16. J Invest Dermatol. 2011. PMID: 21160494 - IL-17-positive mast cell infiltration in the lesional skin of lichen planopilaris: Possible role of mast cells in inducing inflammation and dermal fibrosis in cicatricial alopecia.
Hobo A, Harada K, Maeda T, Uchiyama M, Irisawa R, Yamazaki M, Tsuboi R. Hobo A, et al. Exp Dermatol. 2020 Mar;29(3):273-277. doi: 10.1111/exd.13816. Epub 2018 Dec 28. Exp Dermatol. 2020. PMID: 30379356 - Primary scarring alopecias.
Rigopoulos D, Stamatios G, Ioannides D. Rigopoulos D, et al. Curr Probl Dermatol. 2015;47:76-86. doi: 10.1159/000369407. Epub 2015 Feb 20. Curr Probl Dermatol. 2015. PMID: 26370646 Review.
Cited by
- New Insights into the Role of PPARγ in Skin Physiopathology.
Briganti S, Mosca S, Di Nardo A, Flori E, Ottaviani M. Briganti S, et al. Biomolecules. 2024 Jun 19;14(6):728. doi: 10.3390/biom14060728. Biomolecules. 2024. PMID: 38927131 Free PMC article. Review. - Signaling dynamics and peroxisomes.
Mast FD, Rachubinski RA, Aitchison JD. Mast FD, et al. Curr Opin Cell Biol. 2015 Aug;35:131-6. doi: 10.1016/j.ceb.2015.05.002. Epub 2015 May 29. Curr Opin Cell Biol. 2015. PMID: 26042681 Free PMC article. Review. - Investigating the effects of metabolic dysregulation on hair follicles: a comparison of HIV-infected women with and without central lipohypertrophy.
Mirmirani P, Maurer T, Cohen M, D'Souza G, Karim R, Plankey M, Robison E, Sharma A, Tien PC, Hessol NA. Mirmirani P, et al. Int J Dermatol. 2014 Oct;53(10):e443-8. doi: 10.1111/ijd.12044. Epub 2013 Jun 20. Int J Dermatol. 2014. PMID: 23786579 Free PMC article. - Sterol intermediates of cholesterol biosynthesis inhibit hair growth and trigger an innate immune response in cicatricial alopecia.
Panicker SP, Ganguly T, Consolo M, Price V, Mirmirani P, Honda K, Karnik P. Panicker SP, et al. PLoS One. 2012;7(6):e38449. doi: 10.1371/journal.pone.0038449. Epub 2012 Jun 7. PLoS One. 2012. PMID: 22685570 Free PMC article. - Association of type 2 diabetes with central-scalp hair loss in a large cohort study of African American women.
Coogan PF, Bethea TN, Cozier YC, Bertrand KA, Palmer JR, Rosenberg L, Lenzy Y. Coogan PF, et al. Int J Womens Dermatol. 2019 Jun 6;5(4):261-266. doi: 10.1016/j.ijwd.2019.05.010. eCollection 2019 Sep. Int J Womens Dermatol. 2019. PMID: 31700983 Free PMC article.
References
- Akimoto N, Sato T, Iwata C, Koshizuka M, Shibata F, Nagai A, et al. Expression of perilipin A on the surface of lipid droplets increases along with the differentiation of hamster sebocytes in vivo and in vitro. J Invest Dermatol. 2005;124:1127–1133. - PubMed
- Baeuerle PA, Henkel T. Function and activation of NF-κB in the immune system. Annu Rev Immunol. 1994;12:141–179. - PubMed
- Berger JP, Akiyama TE, Meinke PT. PPARs: therapeutic targets for metabolic disease. Trends Pharmacol Sci. 2005;26:244–251. - PubMed
- Brown WR, Hardy MH. A hypothesis on the cause of chronic epidermal hyperproliferation in asebia mice. Clin Exp Dermatol. 1988;13:74–77. - PubMed
- Cabrero A, Laguna JC, Vazquez M. Peroxisome proliferator-activated receptors and the control of inflammation. Curr Drug Targets Inflamm Allergy. 2002;1:243–248. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30-AR-39750/AR/NIAMS NIH HHS/United States
- P30 AR039750/AR/NIAMS NIH HHS/United States
- P30 AR039750-19/AR/NIAMS NIH HHS/United States
- P30 CA043703/CA/NCI NIH HHS/United States
- P30 AR039750-18/AR/NIAMS NIH HHS/United States
- R01 AR056245/AR/NIAMS NIH HHS/United States
- P30 CA43703/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases