Differential effects of JNK1 and JNK2 inhibition on murine steatohepatitis and insulin resistance - PubMed (original) (raw)
Differential effects of JNK1 and JNK2 inhibition on murine steatohepatitis and insulin resistance
Rajat Singh et al. Hepatology. 2009 Jan.
Abstract
Activation of c-Jun N-terminal kinase (JNK) has been implicated as a mechanism in the development of steatohepatitis. This finding, together with the reported role of JNK signaling in the development of obesity and insulin resistance, two components of the metabolic syndrome and predisposing factors for fatty liver disease, suggests that JNK may be a central mediator of the metabolic syndrome and an important therapeutic target in steatohepatitis. To define the isoform-specific functions of JNK in steatohepatitis associated with obesity and insulin resistance, the effects of JNK1 or JNK2 ablation were determined in developing and established steatohepatitis induced by a high-fat diet (HFD). HFD-fed jnk1 null mice failed to develop excessive weight gain, insulin resistance, or steatohepatitis. In contrast, jnk2(-/-) mice fed a HFD were obese and insulin-resistant, similar to wild-type mice, and had increased liver injury. In mice with established steatohepatitis, an antisense oligonucleotide knockdown of jnk1 decreased the amount of steatohepatitis in concert with a normalization of insulin sensitivity. Knockdown of jnk2 improved insulin sensitivity but had no effect on hepatic steatosis and markedly increased liver injury. A jnk2 knockdown increased hepatic expression of the proapoptotic Bcl-2 family members Bim and Bax and the increase in liver injury resulted in part from a Bim-dependent activation of the mitochondrial death pathway.
Conclusion: JNK1 and JNK2 both mediate insulin resistance in HFD-fed mice, but the JNK isoforms have distinct effects on steatohepatitis, with JNK1 promoting steatosis and hepatitis and JNK2 inhibiting hepatocyte cell death by blocking the mitochondrial death pathway.
Figures
Fig. 1
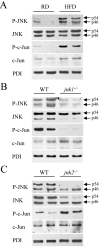
HFD induces hepatic JNK activation and ablation of jnk1 and jnk2 has differential effects on c-Jun phosphorylation. (A) Total protein was isolated from the livers of regular diet (RD)- and HFD-fed mice and immunoblotted with antibodies for phospho-JNK (P-JNK), total JNK, phospho-c-Jun (P-c-Jun) and total c-Jun. Stripped blots were reprobed for PDI. (B) Protein isolated from HFD-fed wild-type (WT) and _jnk1_-/- mice was immunoblotted with the same antibodies. (C) Immunoblots of protein from HFD-fed wild-type and _jnk2_-/- mice. The p54 and p46 forms of JNK are indicated with arrows. Findings are representative of those from 3 independent experiments.
Fig. 2
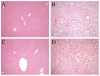
HFD-fed _jnk1_-/- mice have improved histology. Hematoxylin and eosin stained sections of (A) wild-type mice fed a regular diet, (B) wild-type mice fed 16 weeks of a HFD, (C) _jnk1_-/- mice fed a HFD, and (D) HFD-fed _jnk2_-/- mice (magnification 100X).
Fig. 3
HFD-induced steatohepatitis is decreased in _jnk1_-/- mice whereas liver injury increased in _jnk2_-/- mice. (A) Histological steatosis grade in HFD-fed wild-type (WT), _jnk1_-/- and _jnk2_-/- mice (n=7-12; *P<0.0001 compared to wild-type or _jnk2_-/- mice). (B) TG content in the livers of regular diet (RD)- and HFD-fed wild-type, _jnk1_-/- and _jnk2_-/- mice (n=6-15; *P<0.01 compared to RD-fed wild-type mice; **P<0.0003 compared to _jnk1_-/- or jnk2_-/- HFD-fed mice). (C) Serum ALT levels from the same animals (n=5-13; *P<0.001 as compared to RD-fed wild-type mice; **P<0.0001 as compared to HFD-fed wild-type mice; §_P<0.01 when compared to HFD-fed wild-type or _jnk1_-/- mice). (D) Percentage of TUNEL positive cells in the same livers (n=5-9; *P<0.0001 compared to RD-fed wild-type mice; **P<0.00001 compared to wild-type or jnk2_-/- HFD-fed mice; §_P<0.005 compared to HFD-fed wild-type mice). (E) Histological inflammation grade in HFD-fed wild-type, _jnk1_-/- and _jnk2_-/- mice (n=7-12; *P<0.02 compared to wild-type or _jnk2_-/- mice).
Fig. 4
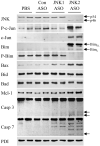
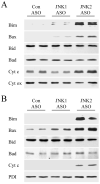
JNK2 ASO treatment of HFD-fed mice results in increased Bim and Bax expression. Total protein isolated from the livers of HFD-fed mice treated with PBS, or control (Con), JNK1 or JNK2 ASO was immunoblotted for total JNK, Bim, Ser65 phospho-Bim (P-Bim), Bax, Bad, Mcl-1, caspase 3 (Casp 3), caspase 7 (Casp 7) or PDI. Arrows indicate the p54 and p46 forms of JNK, BimEL and BimL and the cleaved fragments of caspase 3 and 7. Results are representative of three independent experiments.
Fig. 5
JNK1 and JNK2 ASO treatment of HFD-fed mice has differential effects on insulin resistance, steatosis and liver injury. (A) Fasting serum glucose levels in HFD-fed mice treated with the control (Con), JNK1 or JNK2 ASO (n=7-9; *P<0.0002 compared to control ASO treated; **P<0.00001 as compared to control or JNK1 ASO treated). (B) Fasting serum insulin levels in the same mice (n=7-9; *P<0.02 compared to control ASO-injected mice). (C) HOMA values in the animals (n=7-9; *P<0.01 compared to control ASO treated; **P<0.01 as compared to control or JNK1 ASO treated). (D) Hepatic TG levels (n=7-8; *P<0.04 as compared to wild-type and P<0.004 when compared to JNK2 mice). (E) Percentage of TUNEL positive cells in the same livers along with those from regular diet (RD)-fed mice who did not receive ASO treatment (∅) (n=3-5; *P<0.01 compared to control ASO injected; **P<0.01 compared to control or JNK1 ASO-treated mice).
Fig. 6
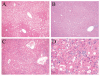
JNK1 ASO treatment decreases steatohepatitis in HFD-fed mice whereas JNK2 ASO treatment worsens liver injury. Hematoxylin and eosin stained sections of HFD-fed mice treated with the control (A), JNK1 (B) or JNK2 (C) ASO (magnification 100X). (D) Higher magnification (400X) of (C).
Fig. 7
Hepatic mitochondrial Bim and Bax translocation and cytochrome c release occur in JNK2 ASO-treated HFD-fed mice. Mitochondrial (A) and cytoplasmic (B) protein fractions were isolated from HFD-fed mice administered a control (Con), JNK1 or JNK2 ASO. Proteins were immunoblotted with antibodies for Bim, Bax, Bid, Bad and cytochrome c (cyt c). Stripped blots were reprobed for cytochrome oxidase (cyt ox) and PDI as indicated.
Fig. 8
Bim null mice are protected from the increase in liver injury and cell death induced by a JNK2 knockdown. (A) Serum ALT levels in HFD-fed wild-type mice (WT) treated with the JNK1 or JNK2 ASO and _bim_-/- mice treated with JNK2 ASO (n=5-12; P<0.0001 as compared to wild-type mice treated with the JNK2 ASO). (B) Numbers of TUNEL positive cells in the same mice (n=6; P<0.00001 as compared to wild-type mice treated with the JNK2 ASO).
Comment in
- c-Jun N-terminal kinase signaling in the pathogenesis of nonalcoholic fatty liver disease: Multiple roles in multiple steps.
Kodama Y, Brenner DA. Kodama Y, et al. Hepatology. 2009 Jan;49(1):6-8. doi: 10.1002/hep.22710. Hepatology. 2009. PMID: 19111006 No abstract available.
Similar articles
- JNK1 but not JNK2 promotes the development of steatohepatitis in mice.
Schattenberg JM, Singh R, Wang Y, Lefkowitch JH, Rigoli RM, Scherer PE, Czaja MJ. Schattenberg JM, et al. Hepatology. 2006 Jan;43(1):163-72. doi: 10.1002/hep.20999. Hepatology. 2006. PMID: 16374858 - c-Jun N-terminal kinase-1 from hematopoietic cells mediates progression from hepatic steatosis to steatohepatitis and fibrosis in mice.
Kodama Y, Kisseleva T, Iwaisako K, Miura K, Taura K, De Minicis S, Osterreicher CH, Schnabl B, Seki E, Brenner DA. Kodama Y, et al. Gastroenterology. 2009 Oct;137(4):1467-1477.e5. doi: 10.1053/j.gastro.2009.06.045. Epub 2009 Jun 21. Gastroenterology. 2009. PMID: 19549522 Free PMC article. - Jnk1 Deficiency in Hematopoietic Cells Suppresses Macrophage Apoptosis and Increases Atherosclerosis in Low-Density Lipoprotein Receptor Null Mice.
Babaev VR, Yeung M, Erbay E, Ding L, Zhang Y, May JM, Fazio S, Hotamisligil GS, Linton MF. Babaev VR, et al. Arterioscler Thromb Vasc Biol. 2016 Jun;36(6):1122-31. doi: 10.1161/ATVBAHA.116.307580. Epub 2016 Apr 21. Arterioscler Thromb Vasc Biol. 2016. PMID: 27102962 Free PMC article. - cJun NH2-terminal kinase 1 (JNK1): roles in metabolic regulation of insulin resistance.
Sabio G, Davis RJ. Sabio G, et al. Trends Biochem Sci. 2010 Sep;35(9):490-6. doi: 10.1016/j.tibs.2010.04.004. Epub 2010 May 7. Trends Biochem Sci. 2010. PMID: 20452774 Free PMC article. Review. - JNK regulation of hepatic manifestations of the metabolic syndrome.
Czaja MJ. Czaja MJ. Trends Endocrinol Metab. 2010 Dec;21(12):707-13. doi: 10.1016/j.tem.2010.08.010. Epub 2010 Oct 1. Trends Endocrinol Metab. 2010. PMID: 20888782 Free PMC article. Review.
Cited by
- Regulation of the effects of CYP2E1-induced oxidative stress by JNK signaling.
Schattenberg JM, Czaja MJ. Schattenberg JM, et al. Redox Biol. 2014;3:7-15. doi: 10.1016/j.redox.2014.09.004. Epub 2014 Sep 23. Redox Biol. 2014. PMID: 25462060 Free PMC article. Review. - Analysis of molecular mechanisms of 5-fluorouracil-induced steatosis and inflammation in vitro and in mice.
Sommer J, Mahli A, Freese K, Schiergens TS, Kuecuekoktay FS, Teufel A, Thasler WE, Müller M, Bosserhoff AK, Hellerbrand C. Sommer J, et al. Oncotarget. 2017 Feb 21;8(8):13059-13072. doi: 10.18632/oncotarget.14371. Oncotarget. 2017. PMID: 28055957 Free PMC article. - How does hepatic lipid accumulation lead to lipotoxicity in non-alcoholic fatty liver disease?
Geng Y, Faber KN, de Meijer VE, Blokzijl H, Moshage H. Geng Y, et al. Hepatol Int. 2021 Feb;15(1):21-35. doi: 10.1007/s12072-020-10121-2. Epub 2021 Feb 6. Hepatol Int. 2021. PMID: 33548031 Free PMC article. Review. - Depletion of cytosolic or mitochondrial thioredoxin increases CYP2E1-induced oxidative stress via an ASK-1-JNK1 pathway in HepG2 cells.
Yang L, Wu D, Wang X, Cederbaum AI. Yang L, et al. Free Radic Biol Med. 2011 Jul 1;51(1):185-96. doi: 10.1016/j.freeradbiomed.2011.04.030. Epub 2011 Apr 22. Free Radic Biol Med. 2011. PMID: 21557999 Free PMC article.
References
- Adams LA, Lindor KD. Nonalcoholic fatty liver disease. Ann Epidemiol. 2007;17:863–869. - PubMed
- Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844–1850. - PubMed
- Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–1192. - PubMed
- Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. - PubMed
- Kaneto H, Nakatani Y, Miyatsuka T, Kawamori D, Matsuoka TA, Matsuhisa M, et al. Possible novel therapy for diabetes with cell-permeable JNK-inhibitory peptide. Nat Med. 2004;10:1128–1132. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK020541/DK/NIDDK NIH HHS/United States
- DK061498/DK/NIDDK NIH HHS/United States
- P30 DK020541/DK/NIDDK NIH HHS/United States
- P60 DK020541/DK/NIDDK NIH HHS/United States
- R01 DK061498/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous