Targeting STAT3 in cancer: how successful are we? - PubMed (original) (raw)
Review
Targeting STAT3 in cancer: how successful are we?
Peibin Yue et al. Expert Opin Investig Drugs. 2009 Jan.
Abstract
Background: Aberrant activation of the signal transducer and activator of transcription (STAT)3 occurs in many human tumors. Moreover, studies utilizing genetic and pharmacological approaches to modulate constitutive STAT3 activity have provided compelling evidence for the critical role of aberrant STAT3 activity in malignant transformation and tumor progression, and thereby validated STAT3 as a novel cancer drug target.
Objective: This review is intended to be a full coverage of the efforts to develop direct STAT3 inhibitors and will provide a discussion on the inhibitory modalities developed to date.
Methods: Review of the literature focused on the modalities and mechanisms that directly target and inhibit the STAT protein or its functions.
Results/conclusion: While a variety of STAT3 inhibitors have been identified that induce antitumor cell effects in vitro and in vivo, the landscape remains murky. With a few exceptions, most of the STAT3 inhibitors reported to date have not undergone an in vivo efficacy, pharmacology or toxicity testing. Also, there is no evidence, per the published literature of an impending clinical development for the few agents that were reported to exhibit in vivo efficacy. Overall, there is the need for a reassessment of the ongoing strategies to target STAT3 intended not only for refinement, but also for incorporating some new technologies to strengthen our efforts and ensure the success - sooner, rather than later - of identifying suitable anti-STAT3 agents for development into clinically useful anticancer therapeutics.
Conflict of interest statement
Declaration of interest The authors state no conflict of interest and have received no payment in preparation of this manuscript.
Figures
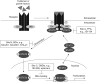
Figure 1. STAT3 signaling pathway and the specific targeting sites for inhibitors
Polypeptides binding to their cognate receptors activates the tyrosine (Tyr) kinase (TK) activities of the receptors, JAKs, or Src. Latent STAT3 is recruited to the activated receptor for phosphorylation (site A) by TKs on critical Tyr residue, leading to STAT3:STAT3 dimerization (site B) and activation. STAT3 accumulates in the nucleus, where it binds to specific DNA response elements (site C) in the promoters of target genes, leading to gene transcription. Currently identified STAT3 inhibitors can block STAT3 biological activity and function via interrogating at specific sites A, B, C, and D (shown in Figure 1 and Figure 2). DBDIs: DNA binding domain inhibitors; JAKs: Janus kinases; P: Phosphate; SDIs: SH2 domain inhibitors or dimerizaton inhibitors; Src: Src family kinases; STAT: Signal transducer and activator of transcription; TPIs: Tyr phosphorylation inhibitors.
Figure 2. Schematic representation of the structural domains of the STAT3 protein
STAT3 protein is modular in structure. The N-terminus contains a domain (ND) that mediates STAT dimer–dimer interactions in tetramer formation that is essential for stabilizing the binding of the dimers to DNA. The coiled-coil domain (CCD) links the ND to the DNA-binding domain (DBD) and participates in the interactions with other proteins. The DBD makes physical contact with STAT3-response elements in the promoters of target genes and is linked to the Src homology 2 (SH2) domain by the linker domain (Linker). The SH2 domain is important for dimerization between two STAT3 monomers. Upon the phosphorylation of the critical Tyr (Y) residue in the transcriptional activation domain (TAD), the SH2 domain of one monomer and the pTyr residue of another engage in reciprocal pTyr-SH2 domain interaction. The transactivation domain mediates the transcriptional activation of target genes and may contain a serine residue essential for maximal transcriptional activity in the case of some STAT proteins, including STAT3. Sites B, C, and D indicate the specific sites where currently identified STAT3 inhibitors interact with the STAT3 protein. DBDIs: DNA binding domain inhibitors; NDIs: N-terminal domain inhibitors; SDIs: SH2 domain inhibitors or dimerizaton inhibitors.
Similar articles
- Small molecule inhibitors of STAT3 for cancer therapy.
Zhao M, Jiang B, Gao FH. Zhao M, et al. Curr Med Chem. 2011;18(26):4012-8. doi: 10.2174/092986711796957284. Curr Med Chem. 2011. PMID: 21824090 Review. - Signal transducer and activator of transcription 3 (STAT3): a promising target for anticancer therapy.
Masciocchi D, Gelain A, Villa S, Meneghetti F, Barlocco D. Masciocchi D, et al. Future Med Chem. 2011 Apr;3(5):567-97. doi: 10.4155/fmc.11.22. Future Med Chem. 2011. PMID: 21526897 Review. - Signal Transducer and Activator of Transcription Protein 3 (STAT3): An Update on its Direct Inhibitors as Promising Anticancer Agents.
Gelain A, Mori M, Meneghetti F, Villa S. Gelain A, et al. Curr Med Chem. 2019;26(27):5165-5206. doi: 10.2174/0929867325666180719122729. Curr Med Chem. 2019. PMID: 30027840 Review. - STAT proteins as novel targets for cancer drug discovery.
Turkson J. Turkson J. Expert Opin Ther Targets. 2004 Oct;8(5):409-22. doi: 10.1517/14728222.8.5.409. Expert Opin Ther Targets. 2004. PMID: 15469392 Review. - STAT3 as a target for inducing apoptosis in solid and hematological tumors.
Al Zaid Siddiquee K, Turkson J. Al Zaid Siddiquee K, et al. Cell Res. 2008 Feb;18(2):254-67. doi: 10.1038/cr.2008.18. Cell Res. 2008. PMID: 18227858 Free PMC article. Review.
Cited by
- Targeting TGF-β signaling in cancer.
Katz LH, Li Y, Chen JS, Muñoz NM, Majumdar A, Chen J, Mishra L. Katz LH, et al. Expert Opin Ther Targets. 2013 Jul;17(7):743-60. doi: 10.1517/14728222.2013.782287. Epub 2013 May 7. Expert Opin Ther Targets. 2013. PMID: 23651053 Free PMC article. Review. - microRNA cluster MC-let-7a-1~let-7d promotes autophagy and apoptosis of glioma cells by down-regulating STAT3.
Yang ZY, Wang Y, Liu Q, Wu M. Yang ZY, et al. CNS Neurosci Ther. 2020 Mar;26(3):319-331. doi: 10.1111/cns.13273. Epub 2019 Dec 23. CNS Neurosci Ther. 2020. PMID: 31868319 Free PMC article. - S1PR1 promotes proliferation and inhibits apoptosis of esophageal squamous cell carcinoma through activating STAT3 pathway.
Liu Y, Zhi Y, Song H, Zong M, Yi J, Mao G, Chen L, Huang G. Liu Y, et al. J Exp Clin Cancer Res. 2019 Aug 22;38(1):369. doi: 10.1186/s13046-019-1369-7. J Exp Clin Cancer Res. 2019. PMID: 31438989 Free PMC article. - The R(h)oads to Stat3: Stat3 activation by the Rho GTPases.
Raptis L, Arulanandam R, Geletu M, Turkson J. Raptis L, et al. Exp Cell Res. 2011 Aug 1;317(13):1787-95. doi: 10.1016/j.yexcr.2011.05.008. Epub 2011 May 18. Exp Cell Res. 2011. PMID: 21619876 Free PMC article. Review. - Targeting EGFR resistance networks in head and neck cancer.
Ratushny V, Astsaturov I, Burtness BA, Golemis EA, Silverman JS. Ratushny V, et al. Cell Signal. 2009 Aug;21(8):1255-68. doi: 10.1016/j.cellsig.2009.02.021. Epub 2009 Mar 1. Cell Signal. 2009. PMID: 19258037 Free PMC article. Review.
References
- Darnell JE., Jr STATs and gene regulation. Science. 1997;277(5332):1630–5. - PubMed
- Turkson J. STAT proteins as novel targets for cancer drug discovery. Expert Opin Ther Targets. 2004;8(5):409–22. - PubMed
- Schroder M, Kroeger KM, Volk HD, et al. Preassociation of nonactivated STAT3 molecules demonstrated in living cells using bioluminescence resonance energy transfer: a new model of STAT activation? J Leukoc Biol. 2004;75(5):792–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous