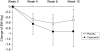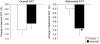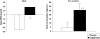Effects of novel capsinoid treatment on fatness and energy metabolism in humans: possible pharmacogenetic implications - PubMed (original) (raw)
Randomized Controlled Trial
Effects of novel capsinoid treatment on fatness and energy metabolism in humans: possible pharmacogenetic implications
Soren Snitker et al. Am J Clin Nutr. 2009 Jan.
Abstract
Background: Capsinoids from the Capsicum genus of plants are nonpungent capsaicin-related substances with effects on metabolism and body weight in animals.
Objectives: Our objectives were to explore the safety and efficacy of capsinoids taken orally (6 mg/d) for weight loss, fat loss, and change in metabolism and to examine whether candidate genes are predictors of capsinoid response.
Design: This was a 12-wk, placebo-controlled, double-blind, randomized study. Eligibility criteria included a body mass index (BMI; in kg/m(2)) of 25-35. Body weight was measured, and dual-energy X-ray absorptiometry, indirect calorimetry (men only), and genotyping were conducted.
Results: Forty women and 40 men with a mean (+/- SD) age of 42 +/- 8 y and BMI of 30.4 +/- 2.4 were randomly assigned to a capsinoid or placebo group. Capsinoids were well tolerated. Mean (+/- SD) weight change was 0.9 +/- 3.1 and 0.5 +/- 2.4 kg in the capsinoid and placebo groups, respectively (P = 0.86). There was no significant group difference in total change in adiposity, but abdominal adiposity decreased more (P = 0.049) in the capsinoid group (-1.11 +/- 1.83%) than in the placebo group (-0.18 +/- 1.94%), and this change correlated with the change in body weight (r = 0.46, P < 0.0001). Changes in resting energy expenditure did not differ significantly between groups, but fat oxidation was higher at the end of the study in the capsinoid group (least-squares mean difference: 21.0 mg/min; P = 0.06). Of 13 genetic variants tested, TRPV1 Val585Ile and UCP2 -866 G/A correlated significantly with change in abdominal adiposity.
Conclusions: Treatment with 6 mg/d capsinoids orally appeared to be safe and was associated with abdominal fat loss. Capsinoid ingestion was associated with an increase in fat oxidation that was nearly significant. We identified 2 common genetic variants that may be predictors of therapeutic response.
Figures
FIGURE 1
Mean (± SE) changes in body weight (BW) from baseline. n = 37 (capsinoids week 0), 33 (capsinoids week 4), 31 (capsinoids weeks 8 and 12), 38 (placebo weeks 0 and 4), 37 (placebo week 8), and 36 (placebo week 12). P = 0.86 for group difference at 12 wk by ANCOVA, adjusted for baseline.
FIGURE 2
Mean (± SE) changes in overall fat and abdominal fat from baseline in the placebo (n = 36) and capsinoid (n = 31) groups. P = 0.67 (overall fat) and *P = 0.049 (abdominal fat) for group difference by ANCOVA, adjusted for baseline.
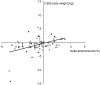
FIGURE 3
Correlation between change in body weight determined by weighing and abdominal fatness determined by dual-energy X-ray absorptiometry in the capsinoid group (solid diamonds; n = 31) and the placebo group (open diamonds; n = 36); r = 0.46, P < 0.0001. The correlation was robust to the removal of the 2 subjects who lost >10 kg body weight (r = 0.29, P = 0.02).
FIGURE 4
Least-squares mean (± SE) changes in resting energy expenditure (REE) and in fat oxidation from baseline in the placebo (n = 16) and capsinoid (n =15) groups. P = 0.19 (REE) and *P = 0.06 (fat oxidation) for group difference by ANCOVA, adjusted for baseline.
Similar articles
- Capsinoids activate brown adipose tissue (BAT) with increased energy expenditure associated with subthreshold 18-fluorine fluorodeoxyglucose uptake in BAT-positive humans confirmed by positron emission tomography scan.
Sun L, Camps SG, Goh HJ, Govindharajulu P, Schaefferkoetter JD, Townsend DW, Verma SK, Velan SS, Sun L, Sze SK, Lim SC, Boehm BO, Henry CJ, Leow MK. Sun L, et al. Am J Clin Nutr. 2018 Jan 1;107(1):62-70. doi: 10.1093/ajcn/nqx025. Am J Clin Nutr. 2018. PMID: 29381803 - Nonpungent capsaicin analogs (capsinoids) increase energy expenditure through the activation of brown adipose tissue in humans.
Yoneshiro T, Aita S, Kawai Y, Iwanaga T, Saito M. Yoneshiro T, et al. Am J Clin Nutr. 2012 Apr;95(4):845-50. doi: 10.3945/ajcn.111.018606. Epub 2012 Feb 29. Am J Clin Nutr. 2012. PMID: 22378725 Clinical Trial. - Effects of dietary supplementation with epigallocatechin-3-gallate on weight loss, energy homeostasis, cardiometabolic risk factors and liver function in obese women: randomised, double-blind, placebo-controlled clinical trial.
Mielgo-Ayuso J, Barrenechea L, Alcorta P, Larrarte E, Margareto J, Labayen I. Mielgo-Ayuso J, et al. Br J Nutr. 2014 Apr 14;111(7):1263-71. doi: 10.1017/S0007114513003784. Epub 2013 Dec 3. Br J Nutr. 2014. PMID: 24299662 Clinical Trial. - Capsaicin as an anti-obesity drug.
Leung FW. Leung FW. Prog Drug Res. 2014;68:171-9. doi: 10.1007/978-3-0348-0828-6_7. Prog Drug Res. 2014. PMID: 24941669 Review. - Capsaicinoids: a spicy solution to the management of obesity?
Tremblay A, Arguin H, Panahi S. Tremblay A, et al. Int J Obes (Lond). 2016 Aug;40(8):1198-204. doi: 10.1038/ijo.2015.253. Epub 2015 Dec 21. Int J Obes (Lond). 2016. PMID: 26686003 Review.
Cited by
- Combined medium-chain triglyceride and chilli feeding increases diet-induced thermogenesis in normal-weight humans.
Clegg ME, Golsorkhi M, Henry CJ. Clegg ME, et al. Eur J Nutr. 2013 Sep;52(6):1579-85. doi: 10.1007/s00394-012-0463-9. Epub 2012 Nov 20. Eur J Nutr. 2013. PMID: 23179202 - Personality factors predict spicy food liking and intake.
Byrnes NK, Hayes JE. Byrnes NK, et al. Food Qual Prefer. 2013 Apr 1;28(1):213-221. doi: 10.1016/j.foodqual.2012.09.008. Epub 2012 Oct 4. Food Qual Prefer. 2013. PMID: 23538555 Free PMC article. - Activation of Human Brown Adipose Tissue by Capsinoids, Catechins, Ephedrine, and Other Dietary Components: A Systematic Review.
Osuna-Prieto FJ, Martinez-Tellez B, Sanchez-Delgado G, Aguilera CM, Lozano-Sánchez J, Arráez-Román D, Segura-Carretero A, Ruiz JR. Osuna-Prieto FJ, et al. Adv Nutr. 2019 Mar 1;10(2):291-302. doi: 10.1093/advances/nmy067. Adv Nutr. 2019. PMID: 30624591 Free PMC article. - Somatosensory innervation of adipose tissues.
Wang Y, Ye L. Wang Y, et al. Physiol Behav. 2023 Jun 1;265:114174. doi: 10.1016/j.physbeh.2023.114174. Epub 2023 Mar 23. Physiol Behav. 2023. PMID: 36965573 Free PMC article. Review. - Synthesis, Antibacterial and Insecticidal Activities of Novel Capsaicin Derivatives Containing a Sulfonic Acid Esters Moiety.
Xie D, Yang Z, Hu X, Wen Y. Xie D, et al. Front Chem. 2022 Jun 14;10:929050. doi: 10.3389/fchem.2022.929050. eCollection 2022. Front Chem. 2022. PMID: 35774861 Free PMC article.
References
- Yazawa S, Suetome N, Okamoto K, Namiki T. Content of capsaicinoids and capsaicinoid-like substances in fruit of pepper (Capsicum annuum L.) hybrids made with “CH-19 Sweet” as a parent. J Jpn Soc Hort Sci 1989;58:601–7
- Kobata K, Todo T, Yazawa S, Iwai K, Watanabe T. Novel capsaicinoid-like substances, capsiate and dihydrocapsiate, form the fruits of a nonpungent cultivar, CH-19 Sweet, of pepper (Capsicum annuum L.). J Agric Food Chem 1998;46:1695–7
- Yazawa S, Yoneda H, Hosokawa M, Fushiki T, Watanabe T. Novel capsaicinoid like substances in the fruits of the non-pungent cultivar “CH-19 Sweet” of pepper (Capsicum annuum). Capsicum and Eggplant Newsletter 2004;23:17–20
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 1997;389:816–24 - PubMed
- Ohnuki K, Haramizu S, Oki K, Watanabe T, Yazawa S, Fushiki T. Administration of capsiate, a non-pungent capsaicin analog, promotes energy metabolism and suppresses body fat accumulation in mice. Biosci Biotechnol Biochem 2001;65:2735–40 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical