Hereditary spastic paraplegia is a novel phenotype for GJA12/GJC2 mutations - PubMed (original) (raw)
Hereditary spastic paraplegia is a novel phenotype for GJA12/GJC2 mutations
Jennifer L Orthmann-Murphy et al. Brain. 2009 Feb.
Abstract
Recessive mutations in GJA12/GJC2, the gene that encodes the gap junction protein connexin47 (Cx47), cause Pelizaeus-Merzbacher-like disease (PMLD), an early onset dysmyelinating disorder of the CNS, characterized by nystagmus, psychomotor delay, progressive spasticity and cerebellar signs. Here we describe three patients from one family with a novel recessively inherited mutation, 99C>G (predicted to cause an Ile>Met amino acid substitution; I33M) that causes a milder phenotype. All three had a late-onset, slowly progressive, complicated spastic paraplegia, with normal or near-normal psychomotor development, preserved walking capability through adulthood, and no nystagmus. MRI and MR spectroscopy imaging were consistent with a hypomyelinating leukoencephalopathy. The mutant protein forms gap junction plaques at cell borders similar to wild-type (WT) Cx47 in transfected cells, but fails to form functional homotypic channels in scrape-loading and dual whole-cell patch clamp assays. I33M forms overlapping gap junction plaques and functional channels with Cx43, however, I33M/Cx43 channels open only when a large voltage difference is applied to paired cells. These channels probably do not function under physiological conditions, suggesting that Cx47/Cx43 channels between astrocytes and oligodendrocytes are disrupted, similar to the loss-of-function endoplasmic reticulum-retained Cx47 mutants that cause PMLD. Thus, GJA12/GJC2 mutations can result in a milder phenotype than previously appreciated, but whether I33M retains a function of Cx47 not directly related to forming functional gap junction channels is not known.
Figures
Fig. 1
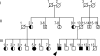
Family pedigree. The family pedigree is shown, and the genotypes were determined for all individuals indicated by asterisks. The proband (III-8; arrowhead), III-11 and III-5 are homozygous for the I33M mutation; II-2, II-9, III-1, III-2, III-3, III-7, and III-12 are asymptomatic heterozygous carriers, and II-9, III-7, and III-12 have normal neurological exams. The other family members were not tested for the mutation and were reported to be neurologically normal. Subjects I-2, I-3 and II-1 had the same surname, suggesting possible consanguinity. Note that unaffected family members of the same gender are grouped together in generation II (e.g. subjects II-3, II-4, II-5 and II-6 are four unaffected males).
Fig. 2
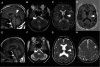
Imaging studies show white matter abnormalities. These are MRI images from patients III-8 (A–D) and III-5 (E–H). Sagittal T1-weighted (T1W) images [spin-echo (SE): repetition time (TR)/echo time (TE) 556/13 ms; 6-mm thickness] shows thinning of the corpus callosum, posteriorly (A, arrowhead) or diffusely (E). Axial T2W images (SE: TR/TE = 3200/90 ms; 5-mm thickness) show symmetric hyperintensity in the region of the corticospinal/corticobulbar tracts at the level of the pons B and F, arrowhead) and the posterior limb of internal capsule (C and G, arrowhead). In addition, there is diffuse hyperintensity in the subcortical, lobar and periventricular white matter (C, G and H), and enlarged ventricles, especially in Patient III-5 (G). Axial T1W image [inversion recovery turbo spin-echo (IRTSE): TR/TE = 5600/70 ms; flip angle 150°; 4-mm thickness] shows diffuse hypointensity in the white matter, including the posterior limb of the internal capsule (D, arrowhead).
Fig. 3
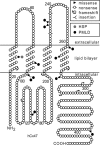
GJA12/GJC2 mutations associated with CNS diseases. This is a schematic drawing of human Cx47, illustrating the position and nature of mutations associated with CNS diseases. I33M (grey circle) is located in the first transmembrane domain. All of the other mutations depicted cause PMLD (black circles), either as homozygous mutations or as compound heterozygote mutations (missense mutations: P87S, G146S, G233R, G233S, T262A, Y269D, M283T and T395I; frameshift mutations: L25fs, P128fs, E204fs, L278fs, P302fs, C315fs, A322fs and P327fs; non-sense mutations: Y229stop and R237stop; complex mutations: A95G____V96insertT) (Uhlenberg et al., 2004; Bugiani et al., 2006; Salviati et al., 2007; Wolf et al., 2007; Henneke et al., 2008). The positions of the transmembrane domains are based on the work of Yeager and Nicholson (Yeager and Nicholson, 1996).
Fig. 4
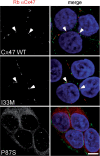
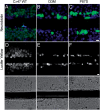
The I33M mutant forms gap junction plaques. These are confocal images of bulk-selected HeLa cells that express WT Cx47 or the indicated mutants, immunostained with a rabbit antiserum against human Cx47 (red) and a mouse monoclonal antibody against pan-cadherin (green), and counterstained with DAPI. The pan-cadherin staining at cell borders interdigitates with the cell surface staining of Cx47 in cells that express WT Cx47 (arrowheads) or I33M (arrowheads), but surrounds the staining of cells expressing the mutant P87S, which is localized in the endoplasmic reticulum. Scale bar: 10 µm.
Fig. 5
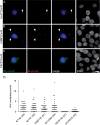
I33M is impermeable to low molecular weight tracers. These are representative images of bulk-selected HeLa cells that stably express WT Cx47 or the indicated mutants, scrape-loaded with neurobiotin (A—C) or Lucifer Yellow (D—F). Cells scrape-loaded with neurobiotin were fixed with 4% paraformaldehyde, then visualized using FITC-conjugated extravidin (green) and DAPI counterstain (blue). Cells scrape-loaded with Lucifer Yellow were imaged by epifluorescence (top row) and phase contrast (bottom row) 5 min after scrape loading. Only HeLa cells expressing WT Cx47 showed transfer of neurobiotin (A) or Lucifer Yellow (D) to neighbouring cells. The images for each type of scrape loading experiment were acquired on the same day at the same exposure. (A—C) scale bar: 20 µm; (D—F) scale bar: 20 µm.
Fig. 6
I33M/Cx43 pairings form gap junction plaques. (A–C) HeLa cells stably expressing Cx47 WT or one of the mutants (I33M or P87S) or Cx43 were transiently transfected to express DsRed (DsRed + ) and mixed with cells expressing one of the connexins, but not transfected with DsRed (DsRed−), in a ratio of 1:20, respectively. After 24 h, cells were immunostained as indicated and counterstained for DAPI. One of the two possible pairings for each combination is illustrated. The DsRed + cell is pseudocoloured blue in the first and third columns, and indicated by an asterisk in the fourth column. Note that I33M/Cx43 (B) pairings have overlapping puncta at the border of the DsRed signal (arrowhead) similar to Cx47/Cx43 pairings (A), whereas P87S/Cx43 pairings (C) do not. (D) Quantitative summary of three independent experiments such as illustrated in (A–C). The asterisk denotes the DsRed + cell. Each dot represents the number of overlapping puncta determined for 1 DsRed + cell. In each column, the horizontal bar denotes the mean, the vertical bar represents the 95% confidence interval, and the total number of DsRed + cells is shown in parentheses. Both pairings of I33M/Cx43 (I33M*/43 and 43*/I33M) have overlapping puncta similar to pairings of Cx47/Cx43 (47*/43 and 43*/47). Results for Cx47/Cx43 and P87S/Cx43 pairings are similar to those previously reported (Orthmann-Murphy et al., 2007_b_).
Fig. 7
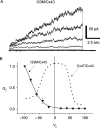
Functional properties of I33M/Cx43 channels. N2A cells were transiently transfected with a pIRES2-EGFP or a pIRES2-DsRed bicistronic expression vector that also contained WT Cx43 or I33M. After 24 h, red and green cell pairs were generated by mixing the transfected cells at a 1 to 1 ratio, and assessed by dual whole-cell patch clamping 24–48 h later. (A) These are representative current traces recorded from an I33M/Cx43 pairing; both cells were voltage clamped to 0 mV and the cell expressing WT Cx43 was stepped in 20 mV increments from _V_j = −100 to _V_j = 100 mV, and junctional current (_I_j) was recorded from the cell expressing I33M. Note that the polarity of _I_j is opposite that of _V_j. Current responses were only activated when a pulse less than or equal to −40 mV was applied to the Cx43-expressing cell. Traces were filtered at 200 Hz. (B) Average normalized _G_j–_V_j relations for heterotypic I33M/Cx43 channels (solid line) and WT Cx47/Cx43 channels (dashed line). For I33M/Cx43 channels, the average _G_j ± SEM at each _V_j (filled triangles) was calculated from current traces such as those shown in (A) and normalized to the value at –100 mV. The WT Cx47/Cx43 channel trace is from Orthmann-Murphy et al., . Note that I33M/Cx43 channels are only open when _V_j is less than −40 mV, and are closed when WT Cx47/Cx43 channels are open (_V_j = 0 mV). Each point in the _G_j–_V_j plot is the average of data from three independent experiments.
Similar articles
- Loss-of-function GJA12/Connexin47 mutations cause Pelizaeus-Merzbacher-like disease.
Orthmann-Murphy JL, Enriquez AD, Abrams CK, Scherer SS. Orthmann-Murphy JL, et al. Mol Cell Neurosci. 2007 Apr;34(4):629-41. doi: 10.1016/j.mcn.2007.01.010. Epub 2007 Jan 25. Mol Cell Neurosci. 2007. PMID: 17344063 Free PMC article. - Activation of the unfolded protein response by Connexin47 mutations associated with Pelizaeus-Merzbacher-like disease.
Flores-Obando RE, Freidin MM, Hernández AI, Abrams CK. Flores-Obando RE, et al. Mol Cell Neurosci. 2022 May;120:103716. doi: 10.1016/j.mcn.2022.103716. Epub 2022 Mar 8. Mol Cell Neurosci. 2022. PMID: 35276347 - Mechanisms of Diseases Associated with Mutation in GJC2/Connexin 47.
Abrams CK. Abrams CK. Biomolecules. 2023 Apr 21;13(4):712. doi: 10.3390/biom13040712. Biomolecules. 2023. PMID: 37189458 Free PMC article. Review. - Gap junctions in inherited human disorders of the central nervous system.
Abrams CK, Scherer SS. Abrams CK, et al. Biochim Biophys Acta. 2012 Aug;1818(8):2030-47. doi: 10.1016/j.bbamem.2011.08.015. Epub 2011 Aug 16. Biochim Biophys Acta. 2012. PMID: 21871435 Free PMC article. Review.
Cited by
- Metabolite profile in hereditary spastic paraplegia analyzed using magnetic resonance spectroscopy: a cross-sectional analysis in a longitudinal study.
Montanaro D, Vavla M, Frijia F, Coi A, Baratto A, Pasquariello R, Stefan C, Martinuzzi A. Montanaro D, et al. Front Neurosci. 2024 Aug 13;18:1416093. doi: 10.3389/fnins.2024.1416093. eCollection 2024. Front Neurosci. 2024. PMID: 39193522 Free PMC article. - An analysis of exome sequencing for diagnostic testing of the genes associated with muscle disease and spastic paraplegia.
Dias C, Sincan M, Cherukuri PF, Rupps R, Huang Y, Briemberg H, Selby K, Mullikin JC, Markello TC, Adams DR, Gahl WA, Boerkoel CF. Dias C, et al. Hum Mutat. 2012 Apr;33(4):614-26. doi: 10.1002/humu.22032. Epub 2012 Feb 28. Hum Mutat. 2012. PMID: 22311686 Free PMC article. - A novel mutation in GJC2 associated with hypomyelinating leukodystrophy type 2 disorder.
Komachali SR, Sheikholeslami M, Salehi M. Komachali SR, et al. Genomics Inform. 2022 Jun;20(2):e24. doi: 10.5808/gi.22008. Epub 2022 Jun 30. Genomics Inform. 2022. PMID: 35794704 Free PMC article. - Lipopolysaccharide induces degradation of connexin43 in rat astrocytes via the ubiquitin-proteasome proteolytic pathway.
Liao CK, Jeng CJ, Wang HS, Wang SH, Wu JC. Liao CK, et al. PLoS One. 2013 Nov 13;8(11):e79350. doi: 10.1371/journal.pone.0079350. eCollection 2013. PLoS One. 2013. PMID: 24236122 Free PMC article. - Novel mutations in the GJC2 gene associated with Pelizaeus-Merzbacher-like disease.
Owczarek-Lipska M, Mulahasanovic L, Obermaier CD, Hörtnagel K, Neubauer BA, Korenke GC, Biskup S, Neidhardt J. Owczarek-Lipska M, et al. Mol Biol Rep. 2019 Aug;46(4):4507-4516. doi: 10.1007/s11033-019-04906-4. Epub 2019 Jul 3. Mol Biol Rep. 2019. PMID: 31270756
References
- Bjartmar C, Yin XH, Trapp BD. Axonal pathology in myelin disorders. J Neurocytol. 1999;28:383–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K02 NS050345-05/NS/NINDS NIH HHS/United States
- R01 NS050705-04/NS/NINDS NIH HHS/United States
- NS050705/NS/NINDS NIH HHS/United States
- R01 NS050705-05/NS/NINDS NIH HHS/United States
- K02 NS050345-04/NS/NINDS NIH HHS/United States
- NS050345/NS/NINDS NIH HHS/United States
- NS55284/NS/NINDS NIH HHS/United States
- R01 NS050705/NS/NINDS NIH HHS/United States
- K02 NS050345/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous