Nolz1 is induced by retinoid signals and controls motoneuron subtype identity through distinct repressor activities - PubMed (original) (raw)
Nolz1 is induced by retinoid signals and controls motoneuron subtype identity through distinct repressor activities
Sheng-Jian Ji et al. Development. 2009 Jan.
Abstract
The acquisition and maintenance of final neuronal identity depends in part upon the implementation of fate-specification programs in postmitotic neurons; however, the mechanisms involved remain unclear. In the developing spinal cord, retinoic acid (RA) signaling pathways specify the columnar and divisional identities of postmitotic motoneurons (MNs). Here we show that RA signals induce expression of the NET transcriptional regulator Nolz1 in differentiated chick MNs, where it regulates the progressive specification of prospective Lim3-negative motor columns. Nolz1 controls the initial formation of forelimb and thoracic Lim3-negative motor columns by downregulating Lim3 expression and maintaining the expression of key homeodomain proteins necessary for MN identity and survival. At forelimb levels, Nolz1 specifies lateral motor column (LMC) identity by inducing the expression of the postmitotic LMC determinant Hoxc6, and implements the partial specification of lateral LMC identity through Lim1 induction. The specificity of Nolz1 function depends upon distinct repressor activities that require, in part, the modulatory activity of Grg5, an atypical member of the Gro-TLE family of co-repressors. Thus, RA signals regulate diverse events in MN subtype specification by inducing the expression of a key transcriptional regulator that controls multiple developmental pathways via functionally distinct repressor complexes.
Figures
Fig. 1.
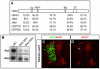
Nolz1 expression is induced by RA signals in spinal cord explants. (A) Conserved domains between chick (c), mouse (m), zebrafish (z) and human (h) predicted Nolz1 ORFs. Sp, Sp1-related domain; FKPY, putative Groucho consensus binding site; Btd, Drosophila Buttonhead domain; ZF, zinc-finger domain. Numbers indicate percentage identity in the regions between these domains. (B) Reverse northern of two different chick Nolz1 EST clones (EST1, EST2) probed with pools of cDNAs generated from St 19 ventral chick embryonic spinal cord explants grown in the presence (+) or absence (-) of retinol (Rol). c29.1 is a cDNA fragment from an unrelated gene known to be transcriptionally unresponsive to RA signals (Rao and Sockanathan, 2005). (C,D) Confocal images of chick spinal cords electroporated with RAR403 showing decreased dorsal Nolz1 expression (asterisk). Motoneurons are reduced as their generation is dependent on RA signaling.
Fig. 2.
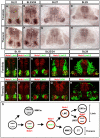
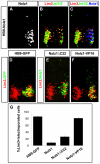
Nolz1 is expressed in subsets of postmitotic spinal MNs. (A-H) In situ hybridization of Nolz1 mRNA expression on transverse sections of embryonic chick spinal cords. (I-R) Confocal micrographs showing analysis of Nolz1 protein expression as compared with molecular markers of specific spinal motor columns. Arrows mark Nolz1 and Lim3 coexpressing MNs. (S) Summary of Nolz1 expression in relation to motor column development. The dashed orange line indicates transient Nolz1 expression. MMC, median motor column; MMCm, medial division of the MMC; MMCl, lateral division of the MMC; LMC, lateral motor column; LMCm, medial division of the LMC; LMCl, lateral division of the LMC; CT, column of Terni.
Fig. 3.
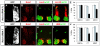
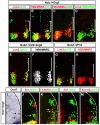
Nolz1 ablation causes a decrease of Lim3- motor columns. (A-D,F-I) Confocal micrographs of sectioned St 23/24 chick spinal cords electroporated with Nolz1 siRNAs and examined for Nolz1 expression and motor column markers by immunohistochemistry at forelimb (FL) and thoracic (T) levels of the spinal cord. (E,J) Bar charts showing the percentage of MNs corresponding to different motor columns in electroporated Nolz1 siRNAs (black bars), control siRNAs (gray bars) and non-electroporated contralateral (white bars, set as 100%) sides of the spinal cord at forelimb (E) and thoracic (J) regions. _n_=4-5 embryos, mean ± s.e.m.
Fig. 4.
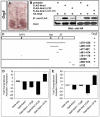
Nolz1 and Grg5 interact and function as transcriptional repressors. (A) In situ hybridization of Grg5 mRNA expression in transverse sections of chick embryonic spinal cords. (B) Co-immunoprecipitation (IP) analyses of Grg5 protein interactions with Nolz1. (C) Schematic of the Nolz1 coding region. FKPY, putative Grg consensus binding site; Btd, Buttonhead domain; ZF, atypical zinc-finger domain. Lines beneath indicate the regions deleted within the Nolz1 coding sequence; the numbers indicate the corresponding amino acids that are deleted. Whether Nolz1 retains (+) or loses (-) its ability to interact with Grg5 in co-IP is indicated alongside. (D) Luciferase-based transcriptional assays using extracts from transfected COS-7 cells. Data are from three replicate experiments, mean ± s.e.m. Student's _t_-test compared with Nolz1: Nolz1+Grg5, _P_=0.0055; Nolz1ΔC22, _P_=0.0016. (E) Luciferase-based transcriptional assays using extracts from electroporated chick spinal cords. _n_=6-8 embryos, mean ± s.e.m. Student's _t_-test compared with Nolz1: Nolz1+Grg5,P<0.000001; Nolz1ΔC22, _P_=0.0029. Compared with GAL4-only: Nolz1+Grg5, _P_=0.0003.
Fig. 5.
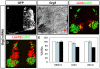
Nolz1 represses Lim3 expression in postmitotic MNs. (A-F) Confocal micrographs of St 21 chick spinal cord sections electroporated on the right. The high levels of Nolz1 elicited by overexpression preclude the detection of endogenous Nolz1 in the spinal cord under these imaging conditions. (G) Bar chart quantifying the percentage of electroporated cells expressing Lim3. _n_=10 embryos, mean ± s.e.m.
Fig. 6.
Nolz1-Grg5 complexes induce postmitotic MNs. Transverse sections of St 21 embryonic chick spinal cords electroporated on the right. White brackets denote ectopic MNs. (A-H,J-M) Immunohistochemical analyses using antibodies against MN and ventral interneuron markers. Arrows indicate ectopic MNs expressing the MN surface marker SC1 (J) or Chx10 (K). (I) Arrows mark ectopic MNs expressing transcripts for choline acetyltransferase (ChAT). Dashed lines mark the lateral extent of the spinal cord.
Fig. 7.
Grg5 knockdown decreases the number of Lim3- motor columns. (A-D) St 24 chick embryos electroporated with Grg5 siRNAs. (A) The right-hand half of the spinal cord is electroporated, as indicated by GFP expression. (B) In situ hybridization of Grg5 mRNA showing efficient ablation of Grg5 mRNA (asterisk) on the Grg5 siRNA-electroporated side. (C,D) Confocal micrographs of sectioned chick spinal cords showing immunohistochemical analysis of motor column markers. (E) Bar chart showing the percentage of motor columns in electroporated Grg5 siRNAs (black bars), control siRNAs (gray bars) and contralateral non-electroporated (white bars, set as 100%) sides of the spinal cord at forelimb regions. _n_=4-5 embryos, mean ± s.e.m.
Fig. 8.
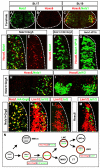
Coexpression of Nolz1 and Grg5 induces Hoxc6 expression and partial lateral LMC specification. (A-C) Confocal micrographs of ventral chick embryonic spinal cords showing that the onset of Nolz1 expression occurs prior to that of Hoxc6. The dashed line outlines the spinal cord. (D-G) Confocal micrographs of electroporated dorsal right halves of St 23 chick spinal cords. Grg5 expression is not shown but is ∼95-98% coincident with exogenous Nolz1. The dashed line marks the lateral boundary of the spinal cord. (H,I) Confocal micrographs of the ventral MN domain of St 23/24 chick embryonic spinal cords electroporated on the right. Yellow cells in panel I mark thoracic MNs that express Hoxc6. (J-M) Confocal micrographs of electroporated dorsal right halves of St 23 chick spinal cords. The dashed line marks the lateral boundary of the spinal cord. The right-hand side is electroporated in all cases. The high levels of Nolz1 elicited by overexpression preclude the detection of endogenous Nolz1 under these imaging conditions. (N) Model for Nolz1-dependent specification of MN subtype identity. Orange lines mark cells expressing Nolz1. Broken orange line refers to transient Nolz1 expression. Nolz1 downregulates Lim3 expression in prospective Lim3-negative MNs and, in a complex with Grg5, maintains the expression of key MN determinants. At limb levels, Nolz1-Grg5 complexes confer forelimb LMC identity through induction of Hoxc6, and induce Lim1 expression in prospective LMCl MNs.
Similar articles
- Regulation of motor neuron subtype identity by repressor activity of Mnx class homeodomain proteins.
William CM, Tanabe Y, Jessell TM. William CM, et al. Development. 2003 Apr;130(8):1523-36. doi: 10.1242/dev.00358. Development. 2003. PMID: 12620979 - Nolz1 promotes striatal neurogenesis through the regulation of retinoic acid signaling.
Urbán N, Martín-Ibáñez R, Herranz C, Esgleas M, Crespo E, Pardo M, Crespo-Enríquez I, Méndez-Gómez HR, Waclaw R, Chatzi C, Alvarez S, Alvarez R, Duester G, Campbell K, de Lera AR, Vicario-Abejón C, Martinez S, Alberch J, Canals JM. Urbán N, et al. Neural Dev. 2010 Aug 24;5:21. doi: 10.1186/1749-8104-5-21. Neural Dev. 2010. PMID: 20735826 Free PMC article. - Genetic and functional modularity of Hox activities in the specification of limb-innervating motor neurons.
Lacombe J, Hanley O, Jung H, Philippidou P, Surmeli G, Grinstein J, Dasen JS. Lacombe J, et al. PLoS Genet. 2013;9(1):e1003184. doi: 10.1371/journal.pgen.1003184. Epub 2013 Jan 24. PLoS Genet. 2013. PMID: 23359544 Free PMC article. - Neuronal development: putting motor neurons in their place.
Guthrie S. Guthrie S. Curr Biol. 2004 Feb 17;14(4):R166-8. Curr Biol. 2004. PMID: 15027472 Review. - AES/GRG5: more than just a dominant-negative TLE/GRG family member.
Beagle B, Johnson GV. Beagle B, et al. Dev Dyn. 2010 Nov;239(11):2795-805. doi: 10.1002/dvdy.22439. Dev Dyn. 2010. PMID: 20925119 Free PMC article. Review.
Cited by
- Identification of two evolutionarily conserved 5' cis-elements involved in regulating spatiotemporal expression of Nolz-1 during mouse embryogenesis.
Chang SL, Liu YC, Chen SY, Huang TH, Liu PT, Liu FC. Chang SL, et al. PLoS One. 2013;8(1):e54485. doi: 10.1371/journal.pone.0054485. Epub 2013 Jan 22. PLoS One. 2013. PMID: 23349903 Free PMC article. - Genetic dissection of photoreceptor subtype specification by the Drosophila melanogaster zinc finger proteins elbow and no ocelli.
Wernet MF, Meier KM, Baumann-Klausener F, Dorfman R, Weihe U, Labhart T, Desplan C. Wernet MF, et al. PLoS Genet. 2014 Mar 13;10(3):e1004210. doi: 10.1371/journal.pgen.1004210. eCollection 2014 Mar. PLoS Genet. 2014. PMID: 24625735 Free PMC article. - Retinoic acid and human olfactory ensheathing cells cooperate to promote neural induction from human bone marrow stromal stem cells.
Xie ST, Lu F, Zhang XJ, Shen Q, He Z, Gao WQ, Hu DH, Yang H. Xie ST, et al. Neuromolecular Med. 2013 Jun;15(2):252-64. doi: 10.1007/s12017-012-8215-9. Epub 2013 Jan 4. Neuromolecular Med. 2013. PMID: 23288654 - Cbln1 regulates axon growth and guidance in multiple neural regions.
Han P, She Y, Yang Z, Zhuang M, Wang Q, Luo X, Yin C, Zhu J, Jaffrey SR, Ji SJ. Han P, et al. PLoS Biol. 2022 Nov 17;20(11):e3001853. doi: 10.1371/journal.pbio.3001853. eCollection 2022 Nov. PLoS Biol. 2022. PMID: 36395107 Free PMC article. - Restricted patterns of Hoxd10 and Hoxd11 set segmental differences in motoneuron subtype complement in the lumbosacral spinal cord.
Misra M, Shah V, Carpenter E, McCaffery P, Lance-Jones C. Misra M, et al. Dev Biol. 2009 Jun 1;330(1):54-72. doi: 10.1016/j.ydbio.2009.03.009. Epub 2009 Mar 21. Dev Biol. 2009. PMID: 19306865 Free PMC article.
References
- Arber, S., Han, B., Mendelsohn, M., Smith, M., Jessell, T. M. and Sockanathan, S. (1999). Requirement for the homeobox gene Hb9 in the consolidation of MN identity. Neuron 23, 659-674. - PubMed
- Chang, C. W., Tsai, C. W., Wang, H. F., Tsai, H. C., Chen, H. Y., Tsai, T. F., Takahashi, H., Li, H. Y., Fann, M. J., Yang, C. W. et al. (2004). Identification of a developmentally regulated striatum-enriched zinc-finger gene, Nolz-1, in the mammalian brain. Proc. Natl. Acad. Sci. USA 101, 2613-2618. - PMC - PubMed
- Dasen, J. S., Liu, J. P. and Jessell, T. M. (2003). MN columnar fate imposed by sequential phases of Hox-c activity. Nature 425, 926-933. - PubMed
- Diez del Corral, R., Olivera-Martinez, I., Goriely, A., Gale, E., Maden, M. and Storey, K. (2003). Opposing FGF and retinoid pathways control ventral neural pattern, neuronal differentiation, and segmentation during body axis extension. Neuron 40, 65-79. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources