Natural-product sugar biosynthesis and enzymatic glycodiversification - PubMed (original) (raw)
Review
Natural-product sugar biosynthesis and enzymatic glycodiversification
Christopher J Thibodeaux et al. Angew Chem Int Ed Engl. 2008.
Abstract
Many biologically active small-molecule natural products produced by microorganisms derive their activities from sugar substituents. Changing the structures of these sugars can have a profound impact on the biological properties of the parent compounds. This realization has inspired attempts to derivatize the sugar moieties of these natural products through exploitation of the sugar biosynthetic machinery. This approach requires an understanding of the biosynthetic pathway of each target sugar and detailed mechanistic knowledge of the key enzymes. Scientists have begun to unravel the biosynthetic logic behind the assembly of many glycosylated natural products and have found that a core set of enzyme activities is mixed and matched to synthesize the diverse sugar structures observed in nature. Remarkably, many of these sugar biosynthetic enzymes and glycosyltransferases also exhibit relaxed substrate specificity. The promiscuity of these enzymes has prompted efforts to modify the sugar structures and alter the glycosylation patterns of natural products through metabolic pathway engineering and enzymatic glycodiversification. In applied biomedical research, these studies will enable the development of new glycosylation tools and generate novel glycoforms of secondary metabolites with useful biological activity.
Figures
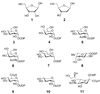
Figure 1. Common sugars of primary metabolism
d
-ribose (1), 2-deoxy-
d
-ribose (2), UDP-
d
-glucose (3), UDP-
d
-galactose (4), UDP-2-_N_-acetyl-
d
-glucosamine (5), UDP-2-_N_-acetyl-
d
-galactosamine (6), GDP-
d
-mannose (7), GDP-
l
-fucose (8), UDP-
d
-glucuronic acid (9), UDP-
d
-xylose (10), CMP-_N_-acetyl-neuraminic acid (sialic acid, 11).
Figure 2. Representative aminoglycoside sugars
The aminosugar substituents of many aminoglycosides contain unusual modifications, whose biosyntheses are not well understood. See text for details.
Figure 3. Selected unusual sugar-tailoring modifications
a) Conversion of
d
-glucose to
d
-mannose (158 → 159) in the biosynthesis of avilamycin by AviX12 – a radical SAM-dependent enzyme. b) Putative function of several radical SAM-dependent enzymes encoded in the neomycin, lividomycin, and paromomycin clusters. c) Methylation at unactivated carbon centers in gentamycin, fortimycin, and moenomycin biosynthesis may be carried out by a group of radical SAM/cobalamin-dependent enzymes. d) Proposed mechanism for Fom3, a radical SAM/cobalamin-dependent enzyme from the fosfomycin biosynthetic pathway of Streptomyces wedmorensis. e) Representative thio-, nitro- and hydroxylamine sugars found in several bacterial natural products. f) Putative involvement of Fms8 in 3,4-didehydroxylation of a fortimycin biosynthetic intermediate and the 2,3,6-trideoxysugar residue (176) found in tobramycin. g) Assembly of the 5-methyl-carboxypyrrole substituent (177) of cloromomycin and coumermycin using non-ribosomal peptide synthase biosynthetic logic. h) Biosynthesis of the unsual sugar substituent (183) in moenomycin. i) Unusual modifications required to synthesize the methyleurekanate moiety (184) of avilamycin A.
Figure 4. Representative bacterial natural product glycoforms
Examples of glycosylated microbial natural products: pikromycin (187), urdamycin A (188), calicheamicin (189), avilamycin (190), BE-7585 (191), vancomycin (192), staurosporine (193), and novobiocin (194). Glycosidic linkages are typically _O_-linked, but _C_-glycosides (see 188), _N_-glycosides (see 193), hydroxylamine linkages (see 189), and orthoester linkages (see 190) also exist in nature. The disaccharide moiety of 191 is coupled to the aromatic aglycone through an unusual thio linkage.
Figure 5. Mechanisms of glycosyltransferases
a) Possible stereochemical outcomes of GT-catalyzed reactions. b) Direct displacement mechanism proposed for inverting GTs. c) Double displacement mechanism proposed for retaining GTs. d) Alternative mechanism for retaining GTs involving nucleophilic attack from the same face of the sugar molecule as leaving group departure. e) Proposed mechanisms for aryl-_C_-glycosidic bond formation. f) UrdGT2 catalyzes the formation of both _C_- and _O_-glycosidic linkages (198 → 199 and 200 → 201, respectively).
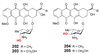
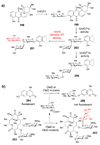
Figure 6. Designed synthesis of an unnatural sugar in Streptomyces peucetius
The axial C4-OH stereochemistry (highlighted in red) of the daunosamine moiety of daunorubicin (202) and doxorubicin (203) was altered to equatorial in 204 and 205 by the replacement of a single S. peucetius
l
-daunosamine biosynthetic gene (dnmV) with 4-ketoreductase genes from
l
-oleandrose (avrE) or
l
-mycarose (eryBIV) biosynthesis.
Figure 7. Engineering erythromycin derivatives in Saccharopolyspora erythraea with gene disruption, heterologous expression, and feeding experiments
Path A – Disruption of individual
l
-mycarose (eryB) or
d
-desosamine (eryC) biosynthetic genes afforded 207–210, 212, and 213. Path B – Heterologous expression of the oleandrosyltransferase (OleG2) from Streptomyces antibioticus in an S. erythraea mutant lacking the endogenous mycarosyltransferase (EryBV). Path C – Expression of the mycaminosyl transferase (TylM2) from the tylosin pathway of Streptomyces fradiae in a triple S. erythraea mutant (SGT2) deficient in desosaminyltransfer (_eryCIII_−), mycarosyltransfer (_eryBV_−), and polyketide synthesis (_eryA_−), generated 216 when the strain was fed tylactone (215). Path D – Novel erythromycin derivates (217 and 218) generated when _O_-methyltransferase genes (spnI and spnK) from Saccharopolyspora spinosa were heterologously expressed in the S. erythraea SGT2 mutant.
Figure 8. Metabolic pathway engineering in the methymycin/pikromycin producer Streptomyces venezuelae
The natural pathway for the biosynthesis of TDP-
d
-desosamine (43) and the glycosylated methymycin/pikromycin derivatives (187, 219–221) produced by S. venezuelae are shown in the boxed pathway. Disruption of individual des genes (highlighted in blue), combined with heterologus expression of foreign genes (highlighted in red) was used to engineer a variety of novel, glycosylated macrolide derivatives (222–232).
Figure 9. Relaxed NDP-sugar substrate specificity of ElmGT, an l-rhamnosyltransferase involved in elloramycin biosynthesis in Streptomyces olivaceus
a) Several naturally occurring aromatic polyketides: urdamycin A (188) produced by Streptomyces fradiae, elloramycin (233) produced by Streptomyces olivaceus, mithramycin (236) produced by Streptomyces argillaceus and the 8-DMTC aglycone (235) encoded by the Streptomyces olivaceus cosmid 16F4. b) Hybrid aromatic polyketides produced in vivo by the action of ElmGT expressed from cosmid 16F4 in the heterologous hosts Streptomyces fradiae (compounds 234 and 237) and Streptomyces argillaceus (compounds 234, 238, and 239). c) Novel aromatic polyketides produced through combinatorial biosynthesis (see text for details).
Figure 10. Manipulating urdamycin biosynthesis
a) Acceptor substrate flexibility of the urdamycin GTs revealed in various Streptomyces fradiae GT mutants. Different combinations of urdamycin A (188) GTs were disrupted, resulting in the production of various glycosylated derivatives (199, 248–258) in the corresponding S. fradiae GT mutant strains. The sugar residues are color-coded to indicate which urdamycin GT is responsible for glycosyl coupling: blue – UdrGT1a, green – UrdGT1b, red – UrdGT1c, and purple – UrdGT2. b) Products isolated from Streptomyces fradiae Tü2717 upon disruption of deoxysugar biosynthetic genes. c) When expressed in glycosyltransferase-deficient mutants of the mithramycin producer Streptomyces argillaceus, UrdGT2 catalyzed production of _C_-glycosides (263 and 264). Heterologous expression of both UrdGT2 and the
d
-olivosyltransferase from the landomycin pathway of Streptomyces cyanogenus (LanGT1) in this same S. argillaceus mutant led to compound 265. Expression of UrdGT2, LanGT1, and LanGT4 in a glycosyltransferase deficient mutant of S. fradiae Tü2717, led to the production of 266.
Figure 10. Manipulating urdamycin biosynthesis
a) Acceptor substrate flexibility of the urdamycin GTs revealed in various Streptomyces fradiae GT mutants. Different combinations of urdamycin A (188) GTs were disrupted, resulting in the production of various glycosylated derivatives (199, 248–258) in the corresponding S. fradiae GT mutant strains. The sugar residues are color-coded to indicate which urdamycin GT is responsible for glycosyl coupling: blue – UdrGT1a, green – UrdGT1b, red – UrdGT1c, and purple – UrdGT2. b) Products isolated from Streptomyces fradiae Tü2717 upon disruption of deoxysugar biosynthetic genes. c) When expressed in glycosyltransferase-deficient mutants of the mithramycin producer Streptomyces argillaceus, UrdGT2 catalyzed production of _C_-glycosides (263 and 264). Heterologous expression of both UrdGT2 and the
d
-olivosyltransferase from the landomycin pathway of Streptomyces cyanogenus (LanGT1) in this same S. argillaceus mutant led to compound 265. Expression of UrdGT2, LanGT1, and LanGT4 in a glycosyltransferase deficient mutant of S. fradiae Tü2717, led to the production of 266.
Figure 11. Novel indolocarbazoles generated by combinatorial biosynthesis in Streptomyces albus
The indolocarbazoles rebeccamycin (267) and staurosporine (193) both contain unusual _N_-glycosidic linkages. Staurosporine biosynthesis was reconstituted in Streptomyces albus by expressing genes required for the formation of the staurosporine aglycone (268), genes encoding production of different deoxysugars (34, 61, 65, 78, and 79), along with the _N_-GT (StaG) and the P450 enzyme (StaN) responsible for the oxidative crosslinking between C5′ of the sugar and the N-12 atom of the aglycone. While StaG coupled both
l
- and
d
-sugars to 268, only the
l
-sugars could be oxidatively crosslinked by StaN to give 193 and 274–277.
Figure 12. Sugar-1-phosphates produced by wild type and engineered E. coli galactokinase (GalK) mutants
The substrate specificity of wild type GalK was broadened by mutation of active site methionine (M173L) and tyrosine (Y371H) residues. The M173L/Y371H double mutant retained the substrate specificity of each single mutant and also accepted a variety of other sugars. The sugar-1-phosphates generated by each enzyme are boxed and the structural deviations from the wild type GalK substrate (
d
-galactose) are highlighted in red.
Figure 13. Protein engineering of glycosyltransferases
a) Novel activity of UrdGT1b/1c chimeras. The biosynthesis of the trisaccharide moiety of urdamycin A involves the tandem action of UrdGT2, UrdGT1c, and UrdGT1b (198 → 199 → 253 → 256). Several chimeric UrdGT1b/1c enzymes catalyzed a new reaction (253 → 281). b) High-throughput screening of GT activity. Random mutagenesis was used to create a library of OleD variants (a macrolide resistance GT that normally catalyzes 282 → 283). The activity of these variants was then screened in a high-throughput fashion using the fluorescent acceptor 284, whose fluorescence is quenched upon glycosyltransfer. Several active mutants were identified in this manner, some of which had broadened substrate specificity (see text for details).
Figure 14. Chemoenzymatic glycorandomization of the vancomycin aglycone
a) The disaccharide moiety of vancomycin (192) is constructed by the tandem addition of
d
-glucose and
l
-vancosamine residues to 286 by the glycosyltransferases GtfE and GtfD, respectively. b) A library of reducing sugars was converted to a library of sugar-1-phosphates by either chemical synthesis or by incubation with engineered GalK mutants and ATP. This library was, in turn, converted into a library of NDP-sugars using engineered RmlA nucleotidylyltransferase. These NDP-sugars were then screened as substrates for GtfE in vitro, leading to a glycorandomized library of 21 vancomycin analogues. One of these analogues contained an azido sugar (see 289) that could be further modified by a variety of acetylene compounds using the CuI-catalyzed Huisgen [3+2] cycloaddition to yield 39 additional vancomycin derivatives.
Figure 15. Exploiting GT reversibility in vitro
a) The calicheamicin GT, CalG1, was shown to synthesize both 293 and 291 from 290 in the presence of TDP through a reverse glycosyltransfer reaction. Alternative TDP-sugars (such as 294) present in the reaction mixture could also be coupled to the aglycone (291) generated in situ to yield a new glycoside, 292. b) CalG1 was used in sugar exchange reactions to rapidly glycorandomize 8 different glycosylated calicheamicin analogues with 10 different sugars to generate a library of 72 compounds. c) One-enzyme aglycone exchange reactions involve a single GT, which transfers a sugar moiety from one aglycone to a structurally similar aglycone. d) Using two enzymes that both recognize the same TDP-sugar, a sugar moiety can be moved from one aglycone to a structurally unrelated aglycone. e) In the presence of excess TDP and a glycosylated natural product, reverse GT catalysis can be used to synthesize TDP-sugars.
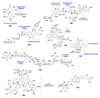
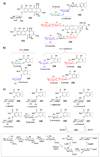
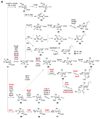
Scheme 1. Biosynthetic origins of NDP-sugars
Most NDP-sugars are derived from glycolytic intermediates glucose-6-phosphate (12) and fructose-6-phosphate (13) or from galactose (16). Eventually, all of these sugars are converted into sugar-1-phosphates, which can then be activated by the appropriate nucleotidylyltransferase.
Scheme 2. Entry point into TDP-deoxysugar secondary metabolism in bacteria
Following thymidylylation of α-
d
-glucose-1-phosphate (17) by a thymidylyltransferase, a TDP-glucose-4,6-dehydratase enzyme catalyzes the conversion of TDP-
d
-glucose (20) to TDP-4-keto-6-deoxy-α-
d
-glucose (21) in the committed step to TDP-deoxysugar biosynthesis.
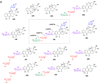
Scheme 3
Scheme 3a: Biosynthesis of Group I TDP-sugars. This group includes 6-deoxysugars (such as 23–28, 30 and 38), as well as the 4-amino-4,6-dideoxysugar (29) and the 3-amino-3,6-dideoxysugar (41). From the common intermediate TDP-4-keto-6-deoxy-α-
d
-glucose (21), most of the TDP-sugars in this group share an epimerization step (21 → 31) early in their biosynthetic pathways. Solid arrows indicate enzyme-catalyzed reactions that have been verified either in vitro through biochemical experiments with purified enzymes or in vivo through gene disruption/heterologous expression experiments. Dashed arrows indicate reactions that have not been experimentally verified, but have been proposed based on comparison of gene sequences to genes of known function. Names in red indicate enzymes whose functions have been verified biochemically using purified enzymes. Scheme 3b: Biosynthesis of Group II TDP-Sugars. The extremely rare TDP-4,6-dideoxysugars include TDP-
d
-desosamine (43), TDP-
d
-chalcomycin (47), and actinospectose (49). The majority of sugars in Group II are 3-amino-2,3,6-trideoxysugars (50–58 and 60–63) that share a common 2-dehydration/3-aminotransfer reaction sequence (21 → 59 → 60). Scheme 3c: Biosynthesis of Group III TDP-sugars. The largest group of TDP-deoxysugars each share 2-dehydration (21 → 59) and 3-ketoreduction steps (59 → 64 or 59 → 73) early in their respective biosynthetic pathways. TDP-sugars derived from 64 are proposed to include 66–72. TDP-sugars proposed to be derived from 73 include numerous 2,6-dideoxysugars (see 75–90) as well as 2,3,6-trideoxysugars (see 91–98), and a TDP-4-amino-2,3,4,6-tetradeoxy sugar (100).
Scheme 3
Scheme 3a: Biosynthesis of Group I TDP-sugars. This group includes 6-deoxysugars (such as 23–28, 30 and 38), as well as the 4-amino-4,6-dideoxysugar (29) and the 3-amino-3,6-dideoxysugar (41). From the common intermediate TDP-4-keto-6-deoxy-α-
d
-glucose (21), most of the TDP-sugars in this group share an epimerization step (21 → 31) early in their biosynthetic pathways. Solid arrows indicate enzyme-catalyzed reactions that have been verified either in vitro through biochemical experiments with purified enzymes or in vivo through gene disruption/heterologous expression experiments. Dashed arrows indicate reactions that have not been experimentally verified, but have been proposed based on comparison of gene sequences to genes of known function. Names in red indicate enzymes whose functions have been verified biochemically using purified enzymes. Scheme 3b: Biosynthesis of Group II TDP-Sugars. The extremely rare TDP-4,6-dideoxysugars include TDP-
d
-desosamine (43), TDP-
d
-chalcomycin (47), and actinospectose (49). The majority of sugars in Group II are 3-amino-2,3,6-trideoxysugars (50–58 and 60–63) that share a common 2-dehydration/3-aminotransfer reaction sequence (21 → 59 → 60). Scheme 3c: Biosynthesis of Group III TDP-sugars. The largest group of TDP-deoxysugars each share 2-dehydration (21 → 59) and 3-ketoreduction steps (59 → 64 or 59 → 73) early in their respective biosynthetic pathways. TDP-sugars derived from 64 are proposed to include 66–72. TDP-sugars proposed to be derived from 73 include numerous 2,6-dideoxysugars (see 75–90) as well as 2,3,6-trideoxysugars (see 91–98), and a TDP-4-amino-2,3,4,6-tetradeoxy sugar (100).
Scheme 3
Scheme 3a: Biosynthesis of Group I TDP-sugars. This group includes 6-deoxysugars (such as 23–28, 30 and 38), as well as the 4-amino-4,6-dideoxysugar (29) and the 3-amino-3,6-dideoxysugar (41). From the common intermediate TDP-4-keto-6-deoxy-α-
d
-glucose (21), most of the TDP-sugars in this group share an epimerization step (21 → 31) early in their biosynthetic pathways. Solid arrows indicate enzyme-catalyzed reactions that have been verified either in vitro through biochemical experiments with purified enzymes or in vivo through gene disruption/heterologous expression experiments. Dashed arrows indicate reactions that have not been experimentally verified, but have been proposed based on comparison of gene sequences to genes of known function. Names in red indicate enzymes whose functions have been verified biochemically using purified enzymes. Scheme 3b: Biosynthesis of Group II TDP-Sugars. The extremely rare TDP-4,6-dideoxysugars include TDP-
d
-desosamine (43), TDP-
d
-chalcomycin (47), and actinospectose (49). The majority of sugars in Group II are 3-amino-2,3,6-trideoxysugars (50–58 and 60–63) that share a common 2-dehydration/3-aminotransfer reaction sequence (21 → 59 → 60). Scheme 3c: Biosynthesis of Group III TDP-sugars. The largest group of TDP-deoxysugars each share 2-dehydration (21 → 59) and 3-ketoreduction steps (59 → 64 or 59 → 73) early in their respective biosynthetic pathways. TDP-sugars derived from 64 are proposed to include 66–72. TDP-sugars proposed to be derived from 73 include numerous 2,6-dideoxysugars (see 75–90) as well as 2,3,6-trideoxysugars (see 91–98), and a TDP-4-amino-2,3,4,6-tetradeoxy sugar (100).
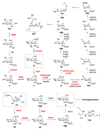
Scheme 4. Biosynthesis of UDP-sugars
a) Biosynthetically, UDP-α-
d
-glucose (3) is derived either from glycolytic intermediate 12, or from β-
d
-galactose (101) via the Leloir pathway (see text for details). The pentose moieties of calicheamicin (104) and avilamycin (107) are likely derived from a UDP-α-
d
-glucose (3) precursor. A separate group of UDP-sugars are derived from the glycolytic intermediate fructose-6-phosphate (13) via UDP-_N_-acetyl-
d
-glucosamine (5, see text for details). Compound 5 is likely the biosynthetic precursor of many aminoglycoside sugars, many of which are 2-aminosugars (see Figure 2).
Scheme 5. Biosynthesis of GDP-sugars
GDP-sugars are derived from GDP-α-
d
-mannose (111), which is in turn derived from fructose-6-phosphate (13, see text for details). The biosynthetic gene clusters for nystatin (nys), amphotericin (amph), pimaricin (pim), candicidin (can), hygromycin A (hyg), avilamycin (avi), and evernimicin (evr) each encode putative GDP-mannose-4,6-dehydratase genes that are predicted to convert 111 to 116. The hygromycin A cluster encodes a putative GDP-6-deoxy-4-keto-
d
-mannose-epimerase/reductase (GMER or GDP-fucose synthase) homologue (Hyg23) -enzymes which are known to convert 116 to 8. The
l
-gulose (114) and 3-_O_-carbamoyl-
d
-mannose (115) residues of bleomycin are proposed to be synthesized from 111 via the GDP-α-
d
-mannose-3,5-epimerase (GME) homologue BlmG and the carbamoyltransferase BlmD, respectively.
Scheme 6. A putative biosynthetic pathway for CDP-l-glucosamine in Streptomyces glaucescens
The biosynthesis of the _N_-methyl-
l
-glucosamine moiety (125) of the aminoglycoside antibiotics streptomycin and bluensomycin is poorly understood. However, the cytidylyltransferase activity (17 → 123) of StrQ encoded by the streptomycin gene cluster of Streptomyces glaucescens was verified in vitro, suggesting that 125 may be derived from a CDP-sugar precursor. The absence of StrQ homologues in other streptomycin and bluensomycin clusters, however, suggests that different biosynthetic routes to 125 may exist (see text for details).
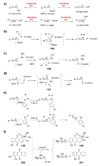
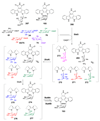
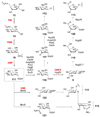
Scheme 7. A common theme in the mechanisms of many deoxysugar biosynthetic enzymes
Many deoxysugar biosynthetic enzymes utilize the 4-keto group installed during the first step of deoxysugar biosynthesis (see 20 → 21, Scheme 2) to catalyze their respective reactions. Some examples include the TDP-4-keto-6-deoxy-
d
-glucose-3,5-epimerase (RmlC) from Pseudomonas aeruginosa (a), the TDP-4-keto-6-deoxy-
d
-glucose-3,4-ketoisomerases (Tyl1a and FdtA) from Streptomyces fradiae and Aneurinibacillus thermoaerophilus, respectively (b), the TDP-4-keto-6-deoxy-
d
-glucose-4-aminotransferase (DesI) from Streptomyces venezuelae (c), the TDP-4-keto-2,6-dideoxy-
d
-glucose-3-_C_-methyltransferase (TylC3) from Streptomyces fradiae (d), the TDP-4-keto-6-deoxy-
d
-glucose-2-dehydratase (TylX3) from S. fradiae (e), the CDP-4-keto-6-deoxy-
d
-glucose-3-dehydrase (E1) from Yersinia pseudotuberculosis (f), and the GDP-4-keto-6-deoxy-
d
-mannose-3-dehydrase (ColD) from Y. pseudotuberculosis (g). See text for mechanistic details of each reaction.
Scheme 7. A common theme in the mechanisms of many deoxysugar biosynthetic enzymes
Many deoxysugar biosynthetic enzymes utilize the 4-keto group installed during the first step of deoxysugar biosynthesis (see 20 → 21, Scheme 2) to catalyze their respective reactions. Some examples include the TDP-4-keto-6-deoxy-
d
-glucose-3,5-epimerase (RmlC) from Pseudomonas aeruginosa (a), the TDP-4-keto-6-deoxy-
d
-glucose-3,4-ketoisomerases (Tyl1a and FdtA) from Streptomyces fradiae and Aneurinibacillus thermoaerophilus, respectively (b), the TDP-4-keto-6-deoxy-
d
-glucose-4-aminotransferase (DesI) from Streptomyces venezuelae (c), the TDP-4-keto-2,6-dideoxy-
d
-glucose-3-_C_-methyltransferase (TylC3) from Streptomyces fradiae (d), the TDP-4-keto-6-deoxy-
d
-glucose-2-dehydratase (TylX3) from S. fradiae (e), the CDP-4-keto-6-deoxy-
d
-glucose-3-dehydrase (E1) from Yersinia pseudotuberculosis (f), and the GDP-4-keto-6-deoxy-
d
-mannose-3-dehydrase (ColD) from Y. pseudotuberculosis (g). See text for mechanistic details of each reaction.
Scheme 8. Mechanisms of selected sugar-modifying short-chain dehydrogenase/reductase (SDR) enzymes
Proposed mechanisms of TDP-α-
d
-glucose-4,6-dehydratase (RmlB) from Salmonella enterica (a), UDP-α-
d
-glucuronate decarboxylase (AviE2) from Streptomyces viridochromogenes (b), UDP-α-
d
-galactose-4-epimerase (GalE) from E. coli (c), GDP-4-keto-6-deoxy-α-
d
-mannose-3,5-epimerase-4-reductase (GMER or GDP-fucose synthase) from E. coli (d), and GDP-α-
d
-mannose-3,5-epimerase (GME) from Arabidopsis thaliana (e). See text for details of each proposed mechanism.
Scheme 9. Enzymatic synthesis of NDP-sugars
a) One-pot synthesis for the common deoxysugar biosynthetic intermediate (TDP-4-keto-6-deoxy-
d
-glucose, 21) from sucrose (280) and thymidine monophosphate (TMP) using TMP kinase (TMK), sucrose synthase (SuSy), and RmlB. b) Biosynthesis-based approach for the synthesis of 21 (see text for details). c) Two-stage, one-pot synthesis of TDP-
l
-mycarose (71). In the first stage, thymidine was converted to TTP by thymidylate kinase (TK), TMK, and nucleotide diphosphate kinase (NDK). Following purification of TTP by filtration, 17 was converted to 71 (in 16% yield from 17) by the combined action of seven enzymes in the presence of _S_-adenosylmethionine (SAM) and NADPH.
Similar articles
- Unusual sugar biosynthesis and natural product glycodiversification.
Thibodeaux CJ, Melançon CE, Liu HW. Thibodeaux CJ, et al. Nature. 2007 Apr 26;446(7139):1008-16. doi: 10.1038/nature05814. Nature. 2007. PMID: 17460661 Review. - [Glycosyl isomerization based on the biosynthesis of natural-product sugar from microorganism].
Sun W, Li HF, Chen J, Wang GJ, Yang ZY. Sun W, et al. Yao Xue Xue Bao. 2013 Feb;48(2):179-86. Yao Xue Xue Bao. 2013. PMID: 23672013 Review. Chinese. - Chapter 12. The power of glycosyltransferases to generate bioactive natural compounds.
Härle J, Bechthold A. Härle J, et al. Methods Enzymol. 2009;458:309-33. doi: 10.1016/S0076-6879(09)04812-5. Methods Enzymol. 2009. PMID: 19374988 - Features and applications of bacterial glycosyltransferases: current state and prospects.
Luzhetskyy A, Bechthold A. Luzhetskyy A, et al. Appl Microbiol Biotechnol. 2008 Oct;80(6):945-52. doi: 10.1007/s00253-008-1672-2. Epub 2008 Sep 6. Appl Microbiol Biotechnol. 2008. PMID: 18777021 Review. - The impact of enzyme engineering upon natural product glycodiversification.
Williams GJ, Gantt RW, Thorson JS. Williams GJ, et al. Curr Opin Chem Biol. 2008 Oct;12(5):556-64. doi: 10.1016/j.cbpa.2008.07.013. Curr Opin Chem Biol. 2008. PMID: 18678278 Free PMC article. Review.
Cited by
- Delineating the biosynthesis of gentamicin x2, the common precursor of the gentamicin C antibiotic complex.
Huang C, Huang F, Moison E, Guo J, Jian X, Duan X, Deng Z, Leadlay PF, Sun Y. Huang C, et al. Chem Biol. 2015 Feb 19;22(2):251-61. doi: 10.1016/j.chembiol.2014.12.012. Epub 2015 Jan 29. Chem Biol. 2015. PMID: 25641167 Free PMC article. - Structural Characterization of CalS8, a TDP-α-D-Glucose Dehydrogenase Involved in Calicheamicin Aminodideoxypentose Biosynthesis.
Singh S, Michalska K, Bigelow L, Endres M, Kharel MK, Babnigg G, Yennamalli RM, Bingman CA, Joachimiak A, Thorson JS, Phillips GN Jr. Singh S, et al. J Biol Chem. 2015 Oct 23;290(43):26249-58. doi: 10.1074/jbc.M115.673459. Epub 2015 Aug 3. J Biol Chem. 2015. PMID: 26240141 Free PMC article. - Cloning and sequencing of the kedarcidin biosynthetic gene cluster from Streptoalloteichus sp. ATCC 53650 revealing new insights into biosynthesis of the enediyne family of antitumor antibiotics.
Lohman JR, Huang SX, Horsman GP, Dilfer PE, Huang T, Chen Y, Wendt-Pienkowski E, Shen B. Lohman JR, et al. Mol Biosyst. 2013 Mar;9(3):478-91. doi: 10.1039/c3mb25523a. Epub 2013 Jan 29. Mol Biosyst. 2013. PMID: 23360970 Free PMC article. - Recombinant E. coli prototype strains for in vivo glycorandomization.
Williams GJ, Yang J, Zhang C, Thorson JS. Williams GJ, et al. ACS Chem Biol. 2011 Jan 21;6(1):95-100. doi: 10.1021/cb100267k. Epub 2010 Oct 22. ACS Chem Biol. 2011. PMID: 20886903 Free PMC article. - Metabolic Engineering of Corynebacterium glutamicum for Production of UDP-N-Acetylglucosamine.
Gauttam R, Desiderato CK, Radoš D, Link H, Seibold GM, Eikmanns BJ. Gauttam R, et al. Front Bioeng Biotechnol. 2021 Sep 23;9:748510. doi: 10.3389/fbioe.2021.748510. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34631687 Free PMC article.
References
- Martin-Rendon E, Blake DJ. Trends Pharmacol. Sci. 2003;24:178. - PubMed
- Durand G, Seta N. Clin. Chem. 2000;46:795. - PubMed
- Este JA. Curr. Med. Chem. 2003;10:1617. - PubMed
- Thorson JS, Hosted TJ, Jr, Jiang J, Biggins JB, Ahlert J. Curr. Org. Chem. 2001;5:139.
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM035906/GM/NIGMS NIH HHS/United States
- GM35906/GM/NIGMS NIH HHS/United States
- GM54306/GM/NIGMS NIH HHS/United States
- R37 GM035906/GM/NIGMS NIH HHS/United States
- R01 GM035906-25/GM/NIGMS NIH HHS/United States
- R01 GM054346-12/GM/NIGMS NIH HHS/United States
- R01 GM054346/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources