Direct observation of a photoinduced radical pair in a cryptochrome blue-light photoreceptor - PubMed (original) (raw)
Direct observation of a photoinduced radical pair in a cryptochrome blue-light photoreceptor
Till Biskup et al. Angew Chem Int Ed Engl. 2009.
No abstract available
Figures
Figure 1
The conserved Trp triad of _Xl_Cry-DASH. (a) Three-dimensional protein structure homology model from the SWISS-MODEL repository (UniProt ID: CRYD_XENLA). (b) Sequence alignment of five members of the photolyase/cryptochrome family. The conserved Trp residues of the putative ET chain in E. coli photolyase (PHR_ECOLI), Syn. Cry-DASH (CRYD_SYNSP), _Xl_Cry-DASH (CRYD_XENLA), garden warbler Cry-1a (CRY1a_GW), and A. thaliana Cry-1 (CRY1_AT) are marked with green triangles. Columns with an alignment score >0.7 are surrounded with a blue frame and the conserved amino acids colored red on white background. If the residues are strictly conserved, they are colored white on red background. The alignment was performed with MultAlin[42] and further processed with ESPript 2.2.[43]
Figure 2
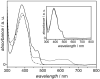
Optical absorption spectra of _Xl_Cry-DASH recorded at 273 K show the FAD cofactor in different oxidation states: FADox (solid line), FADH• (dotted line), and FADH− (dashed line). The inset shows _Xl_Cry-DASH with the FADox cofactor before illumination (solid line), and _Xl_Cry-DASH reoxidized by aerial oxygen after 12 hours of blue-light illumination (dotted line). This is to demonstrate that the protein remains intact in terms of its cofactor contents even upon intensive light illumination conditions.
Figure 3
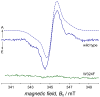
Complete TREPR data set of _Xl_Cry-DASH measured at 274 K. To control for potential shape changes in the TREPR signal caused by gradual sample degradation, spectra were recorded from low to high magnetic field followed by detection in the opposite magnetic-field direction. Each time profile is the average of 120 acquisitions recorded with a laser pulse repetition rate of 1.25 Hz, a microwave frequency of 9.68 GHz, and a power of 2 mW at a detection bandwidth of 100 MHz. A: enhanced absorption; E: emission.
Figure 4
TREPR spectrum of WT (solid blue curve) and W324F (solid green curve) _Xl_Cry-DASH recorded 500 ns after pulsed laser excitation. Experimental parameters were as in Fig. 3. The dashed curve shows a spectral simulation of the WT protein EPR data using the following parameters: gFAD = (2.00431, 2.00360, 2.00217), gTrp = (2.00370, 2.00285, 2.00246), Ω(gFAD⋯gTrp) = (126.5°, 76.5°, 246.5°), D = −0.36 mT, E = 0, Ω(gFAD⋯D) = (0°, 109.9°, 110.5°), J = +0.24 mT.
Similar articles
- Photoreaction of plant and DASH cryptochromes probed by infrared spectroscopy: the neutral radical state of flavoproteins.
Immeln D, Pokorny R, Herman E, Moldt J, Batschauer A, Kottke T. Immeln D, et al. J Phys Chem B. 2010 Dec 30;114(51):17155-61. doi: 10.1021/jp1076388. Epub 2010 Dec 3. J Phys Chem B. 2010. PMID: 21128641 - Ultrafast dynamics of resonance energy transfer in cryptochrome.
Saxena C, Wang H, Kavakli IH, Sancar A, Zhong D. Saxena C, et al. J Am Chem Soc. 2005 Jun 8;127(22):7984-5. doi: 10.1021/ja0421607. J Am Chem Soc. 2005. PMID: 15926801 - Spectroscopic characterization of radicals and radical pairs in fruit fly cryptochrome - protonated and nonprotonated flavin radical-states.
Paulus B, Bajzath C, Melin F, Heidinger L, Kromm V, Herkersdorf C, Benz U, Mann L, Stehle P, Hellwig P, Weber S, Schleicher E. Paulus B, et al. FEBS J. 2015 Aug;282(16):3175-89. doi: 10.1111/febs.13299. Epub 2015 Apr 30. FEBS J. 2015. PMID: 25879256 - Structure and function of animal cryptochromes.
Oztürk N, Song SH, Ozgür S, Selby CP, Morrison L, Partch C, Zhong D, Sancar A. Oztürk N, et al. Cold Spring Harb Symp Quant Biol. 2007;72:119-31. doi: 10.1101/sqb.2007.72.015. Cold Spring Harb Symp Quant Biol. 2007. PMID: 18419269 Review. - Photochemistry and photobiology of cryptochrome blue-light photopigments: the search for a photocycle.
Partch CL, Sancar A. Partch CL, et al. Photochem Photobiol. 2005 Nov-Dec;81(6):1291-304. doi: 10.1562/2005-07-08-IR-607. Photochem Photobiol. 2005. PMID: 16164372 Review.
Cited by
- Decrypting cryptochrome: revealing the molecular identity of the photoactivation reaction.
Solov'yov IA, Domratcheva T, Moughal Shahi AR, Schulten K. Solov'yov IA, et al. J Am Chem Soc. 2012 Oct 31;134(43):18046-52. doi: 10.1021/ja3074819. Epub 2012 Oct 19. J Am Chem Soc. 2012. PMID: 23009093 Free PMC article. - Cryptochrome: A photoreceptor with the properties of a magnetoreceptor?
Ritz T, Yoshii T, Helfrich-Foerster C, Ahmad M. Ritz T, et al. Commun Integr Biol. 2010 Jan;3(1):24-7. doi: 10.4161/cib.3.1.9865. Commun Integr Biol. 2010. PMID: 20539777 Free PMC article. - An open quantum system approach to the radical pair mechanism.
Adams B, Sinayskiy I, Petruccione F. Adams B, et al. Sci Rep. 2018 Oct 24;8(1):15719. doi: 10.1038/s41598-018-34007-4. Sci Rep. 2018. PMID: 30356085 Free PMC article. - Magnetoreception through cryptochrome may involve superoxide.
Solov'yov IA, Schulten K. Solov'yov IA, et al. Biophys J. 2009 Jun 17;96(12):4804-13. doi: 10.1016/j.bpj.2009.03.048. Biophys J. 2009. PMID: 19527640 Free PMC article. - Localisation of the Putative Magnetoreceptive Protein Cryptochrome 1b in the Retinae of Migratory Birds and Homing Pigeons.
Bolte P, Bleibaum F, Einwich A, Günther A, Liedvogel M, Heyers D, Depping A, Wöhlbrand L, Rabus R, Janssen-Bienhold U, Mouritsen H. Bolte P, et al. PLoS One. 2016 Mar 8;11(3):e0147819. doi: 10.1371/journal.pone.0147819. eCollection 2016. PLoS One. 2016. PMID: 26953791 Free PMC article.
References
- Cashmore AR. Cell (Cambridge, Mass) 2003;114:537. - PubMed
- Losi A. Photochem Photobiol. 2007;83:1283. - PubMed
- Brudler R, Hitomi K, Daiyasu H, Toh H, Kucho K-i, Ishiura M, Kanehisa M, Roberts VA, Todo T, Tainer JA, Getzoff ED. Mol Cell. 2003;11:59. - PubMed
- Daiyasu H, Ishikawa T, Kuma K-i, Iwai S, Todo T, Toh H. Genes Cells. 2004;9:479. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources