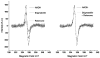Differential effects of mitochondrial Complex I inhibitors on production of reactive oxygen species - PubMed (original) (raw)
Differential effects of mitochondrial Complex I inhibitors on production of reactive oxygen species
Romana Fato et al. Biochim Biophys Acta. 2009 May.
Abstract
We have investigated the production of reactive oxygen species (ROS) by Complex I in isolated open bovine heart submitochondrial membrane fragments during forward electron transfer in presence of NADH, by means of the probe 2',7'-Dichlorodihydrofluorescein diacetate. ROS production by Complex I is strictly related to its inhibited state. Our results indicate that different Complex I inhibitors can be grouped into two classes: Class A inhibitors (Rotenone, Piericidin A and Rolliniastatin 1 and 2) increase ROS production; Class B inhibitors (Stigmatellin, Mucidin, Capsaicin and Coenzyme Q(2)) prevent ROS production also in the presence of Class A inhibitors. Addition of the hydrophilic Coenzyme Q(1) as an electron acceptor potentiates the effect of Rotenone-like inhibitors in increasing ROS production, but has no effect in the presence of Stigmatellin-like inhibitors; the effect is not shared by more hydrophobic quinones such as decyl-ubiquinone. This behaviour relates the prooxidant CoQ(1) activity to a hydrophilic electron escape site. Moreover the two classes of Complex I inhibitors have an opposite effect on the increase of NADH-DCIP reduction induced by short chain quinones: only Class B inhibitors allow this increase, indicating the presence of a Rotenone-sensitive but Stigmatellin-insensitive semiquinone species in the active site of the enzyme. The presence of this semiquinone was also suggested by preliminary EPR data. The results suggest that electron transfer from the iron-sulphur clusters (N2) to Coenzyme Q occurs in two steps gated by two different conformations, the former being sensitive to Rotenone and the latter to Stigmatellin.
Figures
Fig. 1
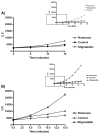
Suitability of DCFDA probe (5 μM) for H2O2 determination in presence of SMP (0.5 mg/ml) supplemented with 150 μM NADH (CTRL) and treated with 1 μM Rotenone (Rotenone). The amount of the deacetylated probe by SMP is largely exceeding that one oxidized by respiratory substrates as indicated by high fluorescence achieved with 5 μM of H2O2. Fluorescence intensity was detected after 2400 s from NADH addition. No fluorescence was detected by addition of 5 μM hydrogen peroxide in absence of SMP. Data are the mean of at least five different determinations±standard deviation.
Fig. 2
ROS production in SMP (0.5 mg/ml) treated with 1.8 μM Mucidin, 10 μM DPI, 75μM CoQ1, 1 μM Rotenone, 1 μM Rotenone plus 75 μM CoQ1 and 1 μM Rotenone plus 50 μM DB. ROS production was detected following the fluorescence variations in presence of 5 μM DCFDA. Each value was detected after 2400 s from 150 μM NADH addition and is expressed as percent of fluorescence change with respect to the control. Data are the mean of at least four different determinations.
Fig. 3
Panel A: Representative experiment of ROS detection in SMP (0.5 mg/ml) inhibited with Class A (i.e. 2 μM Rotenone) and Class B (i.e. 60 μM Stigmatellin) inhibitors. All samples were treated with 1.8 μM Mucidin (to block electron transfer and to avoid ROS production by Complex III) and supplemented with 150 μM NADH. Panel B: As panel A except for SMP concentration (1.5 mg/ml) and Mucidin concentration: 5.4 μM. Inserts show the full time course of the experiments.
Fig. 4
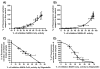
Correlation between percentage of DCFDA fluorescence variation and percentage of NADH–CoQ1 activity inhibition in presence of different Complex I inhibitors: Panel A) Correlation between ROS production and Complex I inhibition by Rotenone. Panel B) Correlation between ROS production and Complex I inhibition by Piericidin A. Samples were prepared as follows: SMP were incubated with increasing amounts of Rotenone or Piericidin A. For each sample we measured NADH:CoQ1 activity and ROS production. Panel C) Effect of increasing amounts of Stigmatellin on ROS produced by 100% Rotenone inhibited Complex I. Panel D) Effect of increasing amount of Stigmatellin on ROS produced by 100% Piericidin A inhibited Complex I. Samples were prepared as follows: SMP completely inhibited with 2 μM of Rotenone or Piericidin A were treated with increasing amounts of Stigmatellin and ROS production was detected. The percentage of inhibition of NADH–CoQ1 activity exerted by the same amounts of Stigmatellin was determined in a parallel experiment. Each value is the mean of at least ten different determinations.
Fig. 5
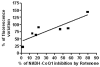
Correlation between ROS production (indicated as percentage of DCFDA fluorescence variation) and percentage of NADH–CoQ1 inhibition by Rotenone in SMP pretreated with 20 pmol/mg of Piericidin A. Addition of 20 pmol/mg of Piericidin A to SMP results in 30% of inhibition of NADH–CoQ1 activity without triggering massive ROS production.
Fig. 6
NADH–DCIP reductase activity related to the physiological quinone reducing site. Data were obtained subtracting the DPI insensitive activity from the total DCIP reductase activity. The stimulation effect of 20 μM DB on NADH–DCIP reductase activity is maintained in presence of Stigmatellin but is abolished by Rotenone. Each value is the mean of at least 5 different determinations. *P<0.005.
Fig. 7
EPR signals of the semiquinone radical in Complex I at 180 K. The systems contained 30 mg/ml of SMP in 300 μl, 150 μM CoQ1. The ubisemiquinone formation was initiated by the addition of 150 μM NADH. Spectra were obtained at a microwave frequency of 9.4121 GHz, obtaining a value of _g_=2.005 for the semiquinone radical.
Fig. 8
Proposed two step mechanism for electron transfer from NADH to quinone in Complex I (A), in presence of Class A inhibitors (B) and in presence of Class B inhibitors (C). The role of hydrophilic (CoQ1) and hydrophobic (DB) quinones is highlighted. CoQ1 can react with the physiological ubiquinone reducing site and, because of its higher water solubility, it can also react with the electron escape site, increasing superoxide production.
Similar articles
- Mitochondrial production of reactive oxygen species: role of complex I and quinone analogues.
Fato R, Bergamini C, Leoni S, Lenaz G. Fato R, et al. Biofactors. 2008;32(1-4):31-9. doi: 10.1002/biof.5520320105. Biofactors. 2008. PMID: 19096098 - Reduction of hydrophilic ubiquinones by the flavin in mitochondrial NADH:ubiquinone oxidoreductase (Complex I) and production of reactive oxygen species.
King MS, Sharpley MS, Hirst J. King MS, et al. Biochemistry. 2009 Mar 10;48(9):2053-62. doi: 10.1021/bi802282h. Biochemistry. 2009. PMID: 19220002 Free PMC article. - Q-site inhibitor induced ROS production of mitochondrial complex II is attenuated by TCA cycle dicarboxylates.
Siebels I, Dröse S. Siebels I, et al. Biochim Biophys Acta. 2013 Oct;1827(10):1156-64. doi: 10.1016/j.bbabio.2013.06.005. Epub 2013 Jun 22. Biochim Biophys Acta. 2013. PMID: 23800966 - Generation of superoxide by the mitochondrial Complex I.
Grivennikova VG, Vinogradov AD. Grivennikova VG, et al. Biochim Biophys Acta. 2006 May-Jun;1757(5-6):553-61. doi: 10.1016/j.bbabio.2006.03.013. Epub 2006 Apr 17. Biochim Biophys Acta. 2006. PMID: 16678117 Review. - Mitochondrial production of oxygen radical species and the role of Coenzyme Q as an antioxidant.
Genova ML, Pich MM, Biondi A, Bernacchia A, Falasca A, Bovina C, Formiggini G, Parenti Castelli G, Lenaz G. Genova ML, et al. Exp Biol Med (Maywood). 2003 May;228(5):506-13. doi: 10.1177/15353702-0322805-14. Exp Biol Med (Maywood). 2003. PMID: 12709577 Review.
Cited by
- Mitochondrial complex I inhibitors, acetogenins, induce HepG2 cell death through the induction of the complete apoptotic mitochondrial pathway.
de Pedro N, Cautain B, Melguizo A, Vicente F, Genilloud O, Peláez F, Tormo JR. de Pedro N, et al. J Bioenerg Biomembr. 2013 Feb;45(1-2):153-64. doi: 10.1007/s10863-012-9489-1. Epub 2012 Nov 21. J Bioenerg Biomembr. 2013. PMID: 23180140 - Mitochondrial involvement and oxidative stress in temporal lobe epilepsy.
Rowley S, Patel M. Rowley S, et al. Free Radic Biol Med. 2013 Sep;62:121-131. doi: 10.1016/j.freeradbiomed.2013.02.002. Epub 2013 Feb 11. Free Radic Biol Med. 2013. PMID: 23411150 Free PMC article. Review. - Cells lacking Rieske iron-sulfur protein have a reactive oxygen species-associated decrease in respiratory complexes I and IV.
Diaz F, Enríquez JA, Moraes CT. Diaz F, et al. Mol Cell Biol. 2012 Jan;32(2):415-29. doi: 10.1128/MCB.06051-11. Epub 2011 Nov 21. Mol Cell Biol. 2012. PMID: 22106410 Free PMC article. - Conformational change of mitochondrial complex I increases ROS sensitivity during ischemia.
Gorenkova N, Robinson E, Grieve DJ, Galkin A. Gorenkova N, et al. Antioxid Redox Signal. 2013 Nov 1;19(13):1459-68. doi: 10.1089/ars.2012.4698. Epub 2013 Mar 29. Antioxid Redox Signal. 2013. PMID: 23419200 Free PMC article. - Complex I generated, mitochondrial matrix-directed superoxide is released from the mitochondria through voltage dependent anion channels.
Lustgarten MS, Bhattacharya A, Muller FL, Jang YC, Shimizu T, Shirasawa T, Richardson A, Van Remmen H. Lustgarten MS, et al. Biochem Biophys Res Commun. 2012 Jun 8;422(3):515-21. doi: 10.1016/j.bbrc.2012.05.055. Epub 2012 May 18. Biochem Biophys Res Commun. 2012. PMID: 22613204 Free PMC article.
References
- Matsuno-Yagi A, Yagi T. Introduction: Complex I—an L-shaped black box. J Bioenerg Biomembr. 2001;33:155–157. - PubMed
- Saraste M. Oxidative phosphorylation at the fin de siecle. Science. 1999;283:1488–1493. - PubMed
- Schultz BE, Chan SI. Structures and proton-pumping strategies of mitochondrial respiratory enzymes. Annu Rev Biophys Biomol Struct. 2001;30:23–65. - PubMed
- Carroll J, Fearnley IM, Shannon RJ, Hirst J, Walker JE. Analysis of the subunit composition of Complex I from bovine heart mitochondria. Mol Cell Proteomics. 2003;2:117–126. - PubMed
- Chomyn A, Cleeter MW, Ragan CI, Riley M, Doolittle RF, Attardi G. URF6, last unidentified reading frame of human mtDNA, codes for an NADH dehydrogenase subunit. Science. 1986;234:614–618. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources