PKD prevents H2O2-induced apoptosis via NF-kappaB and p38 MAPK in RIE-1 cells - PubMed (original) (raw)
PKD prevents H2O2-induced apoptosis via NF-kappaB and p38 MAPK in RIE-1 cells
Jun Song et al. Biochem Biophys Res Commun. 2009.
Abstract
Previously, we demonstrated that protein kinase D (PKD) plays a protective role during H(2)O(2)-induced intestinal cell death. Here, we sought to determine whether this effect is mediated by nuclear factor-kappaB (NF-kappaB) and mitogen-activated protein kinases (MAPKs). Treatment with H(2)O(2) activated NF-kappaB in RIE-1 cells; H(2)O(2) also induced the translocation of NF-kappaB p65 as well as phosphorylation of IkappaB-alpha. PKD1 siRNA inhibited H(2)O(2)-induced activation, translocation of NF-kappaB, and phosphorylation of IkappaB-alpha. We also found that overexpression of wild type PKD1 attenuated H(2)O(2)-induced phosphorylation of p38 MAPK and its upstream activator, MAPK kinase (MKK) 3/6, whereas the phosphorylation was increased by PKD1 siRNA or kinase-dead PKD1. Phosphorylation of neither extracellular signal-regulated kinases (ERK) 1/2 nor c-Jun N-terminal kinases (JNK) was altered by PKD1 plasmids or siRNA. Our findings suggest that PKD protects intestinal cells through up-regulation of NF-kappaB and down-regulation of p38 MAPK.
Figures
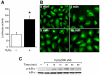
Fig. 1. H2O2 induces the activation of NF-κB, translocation of NF-κB p65 and IκBα phosphorylation in RIE-1 cells
(A) Cells were transfected with 1 μg of a plasmid containing the NF-κB promoter fragment. After 42 h incubation, the transfected cells were treated with H2O2 (100 μM) for 6 h and luciferase activity measured in the cell lysates. The results were normalized for transfection efficiency using the pRL-Tk-luc plasmid [*=p< 0.05 vs. control (−)]. (B) Cells were grown on chamber slides for 2 days then treated with H2O2 (500 μM) or vehicle for 1, 5, 15 min. A representative photomicrograph is shown demonstrating NF-κB p65 localization in using immunofluorescent imaging. (C) After cells were treated with H2O2 (500 μM) over a time course, IκBα phosphorylation was detected by Western blotting using anti-phospho-IκBα (Ser32) antibody. IκBα was used as a loading control.
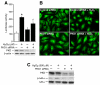
Fig. 2. PKD1 is required for H2O2-stimulated NF-κB activation, translocation and IκBα phosphorylation
(A) RIE-1 cells were co-transfected with a NF-κB plasmid and control siRNA or PKD1 siRNA. After 42 h incubation, the cells were treated with H2O2 for 6 h, and harvested and luciferase activity measured in the cell lysates [*=p< 0.05 vs. control siRNA (−); †=p< 0.05 vs. control siRNA transfected-cells with H2O2 treatment (upper panel)]. PKD expression was examined using anti-PKD antibody (lower panel). (B) RIE-1 cells were transfected with control or PKD1 siRNA and grown on chamber slides for 2 days prior to treatment with H2O2 (500 μM) for 15 min. A representative photomicrograph is shown demonstrating NF-κB localization. (C). Cells were transfected with control or PKD1 siRNA. After 3 days, cells were treated with H2O2 (500 μM) for 15 min and Western blotting was performed. PKD expression was examined using anti-PKD antibody (top row). Phosphorylation of IκBα was detected (middle row) and IκBα was used as a loading control (bottom row).
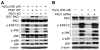
Fig. 3. PKD1 affects H2O2-induced p38 MAPK phosphorylation but not ERK1/2 and JNK>
(A) RIE-1 cells were transfected with PKD1WT, PKD1KD. At 48 h after transfection, cells were treated with H2O2 (500 μM) for 30 min, and protein was extracted for Western blot analysis. PKD overexpression was demonstrated using anti-GST antibody. Phosphorylation of ERK1/2, JNK and p38 MAPK was determined with anti-phospho-ERK1/2, JNK and p38 antibodies. (B) RIE-1 cells were transfected with control or PKD1 siRNA. After 3 days, cells were treated with H2O2 (500 μM) for 30 min, and protein was extracted for Western blotting. Inhibition of PKD expression by PKD1 siRNA was shown with anti-PKD antibody (top row). Phosphorylation of ERK1/2, JNK and p38 MAPK was determined with anti-phospho-ERK1/2, JNK and p38 antibodies. β-actin was used as a loading control.
Fig. 4. Phosphorylation of p38 MAPK induced by H2O2 mediates through MKKs
(A) RIE-1 cells were treated with H2O2 (500 μM) in normal growth medium over a time course; phosphorylation of MKK3/6 was detected by Western blotting (top panel). β-actin was used as a loading control. (B) RIE-1 cells were transfected with PKD1WT, PKD1KD. At 48 h after transfection, cells were treated with H2O2 (500 μM) for 30 min, and protein was extracted for Western blotting. PKD overexpression was demonstrated using anti-PKD antibody (top panel). Phosphorylation of MKK3/6 was determined with anti-phospho-MKK3/6 antibody (second panel). (C) RIE-1 cells were transfected with PKD1 or control siRNA. After 3 days, cells were treated with H2O2 (500 μM) for 30 min, and protein was extracted for Western blotting. Inhibition of PKD expression by PKD1 siRNA was determined using anti-PKD antibody (top panel). Phosphorylation of MKK3/6 was determined with anti-phospho-MKK3/6 antibody (second panel). β-actin was used as a loading control.
Similar articles
- ERK1/2 and p38-MAPK signalling pathways, through MSK1, are involved in NF-kappaB transactivation during oxidative stress in skeletal myoblasts.
Kefaloyianni E, Gaitanaki C, Beis I. Kefaloyianni E, et al. Cell Signal. 2006 Dec;18(12):2238-51. doi: 10.1016/j.cellsig.2006.05.004. Epub 2006 Jun 30. Cell Signal. 2006. PMID: 16806820 - Apoptosis induced by trimethyltin chloride in human neuroblastoma cells SY5Y is regulated by a balance and cross-talk between NF-κB and MAPKs signaling pathways.
Qing Y, Liang Y, Du Q, Fan P, Xu H, Xu Y, Shi N. Qing Y, et al. Arch Toxicol. 2013 Jul;87(7):1273-85. doi: 10.1007/s00204-013-1021-9. Epub 2013 Feb 20. Arch Toxicol. 2013. PMID: 23423712 - Cell stress and MKK6b-mediated p38 MAP kinase activation inhibit tumor necrosis factor-induced IkappaB phosphorylation and NF-kappaB activation.
Alpert D, Schwenger P, Han J, Vilcek J. Alpert D, et al. J Biol Chem. 1999 Aug 6;274(32):22176-83. doi: 10.1074/jbc.274.32.22176. J Biol Chem. 1999. PMID: 10428782 - Transcriptional regulation of lysophosphatidic acid-induced interleukin-8 expression and secretion by p38 MAPK and JNK in human bronchial epithelial cells.
Saatian B, Zhao Y, He D, Georas SN, Watkins T, Spannhake EW, Natarajan V. Saatian B, et al. Biochem J. 2006 Feb 1;393(Pt 3):657-68. doi: 10.1042/BJ20050791. Biochem J. 2006. PMID: 16197369 Free PMC article.
Cited by
- PKD1 alleviates oxidative stress-inhibited osteogenesis of rat bone marrow-derived mesenchymal stem cells through TAZ activation.
Chen T, Wang H, Jiang C, Lu Y. Chen T, et al. J Cell Biochem. 2021 Nov;122(11):1715-1725. doi: 10.1002/jcb.30124. Epub 2021 Aug 18. J Cell Biochem. 2021. PMID: 34407229 Free PMC article. - Protein kinase D signaling: multiple biological functions in health and disease.
Rozengurt E. Rozengurt E. Physiology (Bethesda). 2011 Feb;26(1):23-33. doi: 10.1152/physiol.00037.2010. Physiology (Bethesda). 2011. PMID: 21357900 Free PMC article. Review. - Function and Regulation of Protein Kinase D in Oxidative Stress: A Tale of Isoforms.
Cobbaut M, Van Lint J. Cobbaut M, et al. Oxid Med Cell Longev. 2018 Apr 26;2018:2138502. doi: 10.1155/2018/2138502. eCollection 2018. Oxid Med Cell Longev. 2018. PMID: 29854077 Free PMC article. Review. - Protein kinase D1 - A targetable mediator of pancreatic cancer development.
Fleming Martinez AK, Storz P. Fleming Martinez AK, et al. Biochim Biophys Acta Mol Cell Res. 2024 Feb;1871(2):119646. doi: 10.1016/j.bbamcr.2023.119646. Epub 2023 Dec 5. Biochim Biophys Acta Mol Cell Res. 2024. PMID: 38061566 Review. - Comparative genomics reveals convergent signals associated with the high metabolism and longevity in birds and bats.
Matsuda Y, Makino T. Matsuda Y, et al. Proc Biol Sci. 2024 Aug;291(2029):20241068. doi: 10.1098/rspb.2024.1068. Epub 2024 Aug 28. Proc Biol Sci. 2024. PMID: 39191281 Free PMC article.
References
- Gabbita SP, Robinson KA, Stewart CA, Floyd RA, Hensley K. Redox regulatory mechanisms of cellular signal transduction. Arch Biochem Biophys. 2000;376:1–13. - PubMed
- Martindale JL, Holbrook NJ. Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol. 2002;192:1–15. - PubMed
- Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109(Suppl):S81–96. - PubMed
- Li N, Karin M. Is NF-kappaB the sensor of oxidative stress? Faseb J. 1999;13:1137–1143. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK61470/DK/NIDDK NIH HHS/United States
- R01 DK061470/DK/NIDDK NIH HHS/United States
- R01 CA104748-05/CA/NCI NIH HHS/United States
- P01 DK035608-220001/DK/NIDDK NIH HHS/United States
- R01 DK048498/DK/NIDDK NIH HHS/United States
- R01 DK048498-13/DK/NIDDK NIH HHS/United States
- P01 DK035608/DK/NIDDK NIH HHS/United States
- R01 DK48498/DK/NIDDK NIH HHS/United States
- R01 DK061470-05/DK/NIDDK NIH HHS/United States
- R01 CA104748/CA/NCI NIH HHS/United States
- P01 DK 35608/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous