The actin-cytoskeleton linker protein ezrin is regulated during osteosarcoma metastasis by PKC - PubMed (original) (raw)
The actin-cytoskeleton linker protein ezrin is regulated during osteosarcoma metastasis by PKC
L Ren et al. Oncogene. 2009.
Abstract
Ezrin is a member of the ERM (ezrin, radixin, moesin) protein family and links F-actin to the cell membrane following phosphorylation. Ezrin has been associated with tumor progression and metastasis in several cancers including the pediatric solid tumors, osteosarcoma and rhabdomyosarcoma. In this study, we were surprised to find that ezrin was not constitutively phosphorylated but rather was dynamically regulated during metastatic progression in osteosarcoma. Metastatic osteosarcoma cells expressed phosphorylated ERM early after their arrival in the lung, and then late in progression, only at the invasive front of larger metastatic lesions. To pursue mechanisms for this regulation, we found that inhibitors of PKC (protein kinase C) blocked phosphorylation of ezrin, and that ezrin coimmunoprecipitated in cells with PKCalpha, PKCiota and PKCgamma. Furthermore, phosphorylated forms of ezrin and PKC had identical expression patterns at the invasive front of pulmonary metastatic lesions in murine and human patient samples. Finally, we showed that the promigratory effects of PKC were linked to ezrin phosphorylation. These data are the first to suggest a dynamic regulation of ezrin phosphorylation during metastasis and to connect the PKC family members with this regulation.
Figures
Figure 1
Phosphorylation (activation) of ERM proteins is dynamically regulated during metastatic progression. Metastatic lung nodules were evaluated by immunohistochemistry for expression of phosphorylated ERM and total ezrin. Samples were derived after tail vein injection of K7M2 murine osteosarcoma cells (a–g and j) or MNNG/HOS human osteosarcoma cells (h and k), and from human patient tissue samples (i and l). K7M2 lung metastases were examined at days 5, 15, 20 and 24 after injection (a–g and j). A similar pattern of immunoreactivity for ezrin and phosphorylated ERM were seen in K7M2 pulmonary metastases derived from spontaneous lung metastasis from an appendicular primary tumor in mice (data not shown). Total ezrin and phosphorylated ERM were stained on adjacent lung sections. a and d, Bar = 40 μm; b, c, e and f, bar = 100 μm; g–l, bar = 200 μm.
Figure 2
Ezrin (T567) phosphorylation is dependent on protein kinase C (PKC). (a) Murine osteosarcoma K7M2 cells were incubated with various concentrations of PKC inhibitor BIM, Rho kinase inhibitor Y27632, PI3 kinase inhibitor LY294002 and MEK inhibitor U0126. Phospho-ERM expression is shown by western blot analysis (phosphorylated ERM is comprised of a phospho-ezrin/radixin band * and a phospho-moesin band **). The blots were probed for β-actin as the loading control, and phospho-Akt (Y374) or phospho-MAPK 42/44. (b) K7M2 and MNNG/HOS cells were treated with PKC inhibitors BIM, Ro 31–8220 or Gö 6976 at various concentrations for 60min or for different time periods as indicated. The level of phospho-ERM was analysed by western blotting. (c) Imuunofluorescent detection of phospho-ERM in untreated K7M2 cells or in K7M2 cells treated with 5 μM of Ro 31–8220 for 60min. Bar = 20 μm in low magnification photographs and 5 μm in high magnification photographs.
Figure 3
Ezrin forms protein–protein complexes with PKCα, ι and γ in osteosarcoma cells. (a) Western blot analysis shows expression of the PKC isoforms in K12, K7M2 and MNNG/HOS osteosarcoma cell lines. PKCβ, η and θ isoforms are not expressed in osteosarcoma cells, although they are expressed in Jurkat cells or lung tissue. (b) Immunoprecipitated ezrin from K7M2 or MNNG/ HOS cell extracts were probed for pan-PKC, PKCα, γ, δ, ι, ε, ζ and ezrin. Ezrin interactions were detected with PKC isoforms α, γ and ι. Normal rabbit serum was used as the negative control.
Figure 4
Matching expression patterns of phospho-ERM and phospho-PKCα in metastatic lesions. Immunohistochemistry detection of phosphorylated ERM and phospho-PKCα in K7M2 murine osteosarcoma lung nodules (harvested 24 days after cell injection to mice). Adjacent lung sections were labeled with antibodies against either phospho-PKCα (Ser 657) (a–c) or phospho-ERM (d–f). Phospho-PKCα (a and b) and phospho-ERM (d and e) immunoreactivity was evident at the periphery of metastatic lesions. Both active PKCα expression and phosphorylated ERM expression was low in the central portions of the metastatic lesions (Figures 4c and f). Bar = 200 μm in low magnification photographs and 50 μm in high magnification photographs.
Figure 5
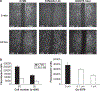
Suppression of ezrin or inhibition of PKC decreases osteosarcoma cell migration. (a) Nearly confluent wild-type ezrin (K7M2) or antisense mediated ezrin knockdown cells (As 1.46) were ‘wounded’ using a P-200 pipette, and images of the denuded area were taken at 0 and 24h. Migration of cells with suppression of ezrin (As1.46) was markedly decreased at 24h compared to K7M2 control cells (high ezrin). K7M2 cells that had been treated with 1 μM PKC inhibitor Gö 6976 also showed a significant reduction in migration. (b) Ezrin knock down (As1.46) and control K7M2 cells were seeded on transwell plates in complete medium (left panel). After an 18-h incubation, cells migrating to the lower chamber were stained with Calcein AM and the fluorescent intensity was quantitated. Ezrin knockdown cells (As1.46) had a marked decrease in migration compared to control cells (K7M2) using either 5 × 104 cells (P = 0.004) or 2.5 × 104 cells (P = 0.01) as starting material. Similarly, K7M2 cells were seeded on transwell plates with 0.1% dimethylsulfoxide or PKC inhibitor Gö 6976 (right panel). Cell migration was inhibited in the presence of Gö 6976 at 0.1 μM (P = 0.05) and at 1 μM (P = 0.03).
Figure 6
PKC-mediated cell migration is in part dependent on ezrin. Expression of constitutively activated phospho-mimetic ezrin mutant (T567D-GFP) in K7M2 osteosarcoma cells allowed association between PKC, ezrin and cell migration to be assessed. (a) Expression of endogenous ezrin and GFP-tagged ezrin mutant proteins in K7M2 parental cells were resolved by western blot analysis and with an anti-ezrin antibody. Lane 1, GFP transfectants; lanes 2–4, three different clones of EzrinT567D-GFP transfectants. (b) Localization of GFP and EzrinT567D-GFP in K7M2 cells was assessed by GFP fluorescence. Whereas GFP was distributed throughout the cytoplasm and nucleus, EzrinT567D-GFP (active ezrin) was discretely localized to the cell membrane with F-actin. (c) Cell migration (wound healing) assay using K7M2 control, GFP control, and three different clones of EzrinT567D-GFP cells (T567D1, T567D3 and T567D2.4) before and after 24h of treatment with either 0.1% dimethylsulfoxide or 0.1 μM Gö 6976. The constitutive activation of ezrin in the EzrinT567D-GFP prevented PKC inhibitors from suppressing osteosarcoma cell migration.
Similar articles
- Protein kinase C regulates ezrin-radixin-moesin phosphorylation in canine osteosarcoma cells.
Hong SH, Osborne T, Ren L, Briggs J, Mazcko C, Burkett SS, Khanna C. Hong SH, et al. Vet Comp Oncol. 2011 Sep;9(3):207-18. doi: 10.1111/j.1476-5829.2010.00249.x. Epub 2010 Dec 14. Vet Comp Oncol. 2011. PMID: 21848623 Free PMC article. - Ezrin and moesin expression in canine and feline osteosarcoma.
Hlavaty J, Wolfesberger B, Hauck M, Obermayer-Pietsch B, Fuchs-Baumgartinger A, Miller I, Walter I. Hlavaty J, et al. Histol Histopathol. 2017 Aug;32(8):805-816. doi: 10.14670/HH-11-848. Epub 2016 Nov 30. Histol Histopathol. 2017. PMID: 27900754 - Role of ezrin in osteosarcoma metastasis.
Ren L, Khanna C. Ren L, et al. Adv Exp Med Biol. 2014;804:181-201. doi: 10.1007/978-3-319-04843-7_10. Adv Exp Med Biol. 2014. PMID: 24924175 Review. - Phosphorylated ezrin is located in the nucleus of the osteosarcoma cell.
Di Cristofano C, Leopizzi M, Miraglia A, Sardella B, Moretti V, Ferrara A, Petrozza V, Della Rocca C. Di Cristofano C, et al. Mod Pathol. 2010 Jul;23(7):1012-20. doi: 10.1038/modpathol.2010.77. Epub 2010 Mar 26. Mod Pathol. 2010. PMID: 20348881 - Osteosarcoma metastasis: prospective role of ezrin.
Zhang Y, Zhang L, Zhang G, Li S, Duan J, Cheng J, Ding G, Zhou C, Zhang J, Luo P, Cai D, Kuang L, Zhou Y, Tong L, Yu X, Zhang L, Xu L, Yu L, Shi X, Ke A. Zhang Y, et al. Tumour Biol. 2014 Jun;35(6):5055-9. doi: 10.1007/s13277-014-1799-y. Epub 2014 Mar 9. Tumour Biol. 2014. PMID: 24609902 Review.
Cited by
- Six3 and Six6 jointly control diverse target genes in multiple cell populations over developmental trajectories of mouse embryonic retinal progenitor cells.
Ferrena A, Zhang X, Shrestha R, Zheng D, Liu W. Ferrena A, et al. PLoS One. 2024 Oct 24;19(10):e0308839. doi: 10.1371/journal.pone.0308839. eCollection 2024. PLoS One. 2024. PMID: 39446806 Free PMC article. - Targeting Mechanotransduction in Osteosarcoma: A Comparative Oncology Perspective.
Luu AK, Viloria-Petit AM. Luu AK, et al. Int J Mol Sci. 2020 Oct 14;21(20):7595. doi: 10.3390/ijms21207595. Int J Mol Sci. 2020. PMID: 33066583 Free PMC article. Review. - Apoptosis resistance and PKC signaling: distinguishing features of high and low metastatic cells.
Hong SH, Ren L, Mendoza A, Eleswarapu A, Khanna C. Hong SH, et al. Neoplasia. 2012 Mar;14(3):249-58. doi: 10.1593/neo.111498. Neoplasia. 2012. PMID: 22496624 Free PMC article. - Initiation of malignancy by duodenal contents reflux and the role of ezrin in developing esophageal squamous cell carcinoma.
Ling ZQ, Mukaisho K, Yamamoto H, Chen KH, Asano S, Araki Y, Sugihara H, Mao WM, Hattori T. Ling ZQ, et al. Cancer Sci. 2010 Mar;101(3):624-30. doi: 10.1111/j.1349-7006.2009.01470.x. Epub 2009 Dec 15. Cancer Sci. 2010. PMID: 20128822 Free PMC article. - Stable knockdown of S100A4 suppresses cell migration and metastasis of osteosarcoma.
Fujiwara M, Kashima TG, Kunita A, Kii I, Komura D, Grigoriadis AE, Kudo A, Aburatani H, Fukayama M. Fujiwara M, et al. Tumour Biol. 2011 Jun;32(3):611-22. doi: 10.1007/s13277-011-0160-y. Epub 2011 Mar 1. Tumour Biol. 2011. PMID: 21360024
References
- Berryman M, Franck Z, Bretscher A. (1993). Ezrin is concentrated in the apical microvilli of a wide variety of epithelial cells whereas moesin is found primarily in endothelial cells. J Cell Sci 105(Part 4): 1025–1043. - PubMed
- Blobe GC, Obeid LM, Hannun YA. (1994). Regulation of protein kinase C and role in cancer biology. Cancer Metastasis Rev 13: 411–431. - PubMed
- Bornancin F, Parker PJ. (1997). Phosphorylation of protein kinase C-alpha on serine 657 controls the accumulation of active enzyme and contributes to its phosphatase-resistant state. J Biol Chem 272: 3544–3549. - PubMed
- Bretscher A, Chambers D, Nguyen R, Reczek D. (2000). ERM-Merlin and EBP50 protein families in plasma membrane organization and function. Annu Rev Cell Dev Biol 16: 113–143. - PubMed
- Bretscher A, Edwards K, Fehon RG. (2002). ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol 3: 586–599. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials