TLR-independent type I interferon induction in response to an extracellular bacterial pathogen via intracellular recognition of its DNA - PubMed (original) (raw)
TLR-independent type I interferon induction in response to an extracellular bacterial pathogen via intracellular recognition of its DNA
Marie Charrel-Dennis et al. Cell Host Microbe. 2008.
Abstract
Type I interferon (IFN) is an important host defense cytokine against intracellular pathogens, mainly viruses. In assessing IFN production in response to group B streptococcus (GBS), we find that IFN-beta was produced by macrophages upon stimulation with both heat-killed and live GBS. Exposure of macrophages to heat-killed GBS activated a Toll-like receptor (TLR)-dependent pathway, whereas live GBS activated a TLR/NOD/RIG-like receptor (RLR)-independent pathway. This latter pathway required bacterial phagocytosis, proteolytic bacterial degradation, and phagolysosomal membrane destruction by GBS pore-forming toxins, leading to the release of bacterial DNA into the cytosol. GBS DNA in the cytosol induced IFN-beta production via a pathway dependent on the activation of the serine-threonine kinase TBK1 and phosphorylation of the transcription factor IRF3. Thus, activation of IFN-alpha/-beta production during infection with GBS, commonly considered an extracellular pathogen, appears to result from the interaction of GBS DNA with a putative intracellular DNA sensor or receptor.
Figures
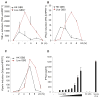
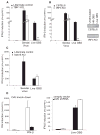
Figure 1. Live and Heat-Killed GBS Organisms Induce Type I IFNs and Proinflammatory Cytokines
(A–C) WT bone marrow-derived macrophages (BMDMs) were stimulated for the indicated amount of time with heat-killed GBS (HK GBS; 80 μg/ml) or live GBS (MOI 6:1). Levels of mRNA for TNF-α (A), _IFN_-β (B), and viperin (C) were determined by Q-PCR and normalized to HPRT1 level of expression. (D) WT BMDMs were stimulated for 12 hr with two-fold decreasing amounts of bacteria; the initial concentration of heat-killed GBS (HK GBS; 80 μg/ml), live GBS (MOI 10:1) and Sendai virus (150 HAU/ml). IFN-β was detected by ELISA. *, P < 0.05, Student’s t test (all panels).
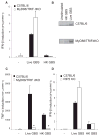
Figure 2. Live and Heat-Killed GBS Bacteria Activate Distinct Pathways Leading to Type I IFNs Production
(A–C) WT and MyD88/TRIF-deficient BMDM were stimulated as in Figure 1 for 6 hr and the amount of IFN-β (A) and TNF-α (C) mRNA determined by Q-PCR and were normalized to HPRT1 level of expression. (B) Phosphorylation of the transcription factor STAT2 in WT or MyD88/TRIF-deficient BMDMs was compared after 2 hr infection with live GBS (MOI 10:1). *, P < 0.05, Student’s t test. (D) WT and RIP2-deficient BMDMs were stimulated as mentioned above and the amount of IFN-β mRNA determined.
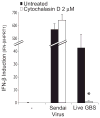
Figure 3. Phagocytosis of Live GBS Is Required for the Release of the IFN-Inducing Material
WT BMDMs were pretreated for 30 min with cytochalasin D (2 μM) before being infected with live GBS (MOI 6:1) for 6 hr or Sendai virus (150 HAU/ml) for 4 hr and IFN-β mRNA measured as in Figure 1. *, P < 0.05, Student’s t test.
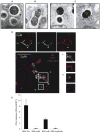
Figure 4. A Small Proportion of Live GBS Bacteria Escapes the Phagosome and Reaches the Cytosol upon Phagosomal Membranes Degradation
(A–C) Electron microscopy of WT BMDMs infected for 2 hr with GBS. Most GBS organisms were found in the phagosome (A), but a few were observed escaping the phagosome (B), and some were ultimately identified in the cytosol (C). The black arrow shows the phagosomal membrane, whereas the white arrow highlights the absence of phagosomal membranes. The magnification is 39,000×. (D) Confocal microscopy of WT BMDMs infected with live GBS (MOI 10:1) for 2 hr. The phagosomal membrane is stained with Lamp1 (red) and the surface of the bacteria with an anti-Group B antigen antibody (green). The white arrow shows bacteria located outside the Lamp1-stained membrane. In the lower panel, the plasma and phagosomal membranes were also stained with cholera toxin b (blue). Phagocytosed bacteria were found in the phagosomal compartment (A) and outside of this compartment but within the confines of the plasma membrane (B). (E) WT C57BL/6 BMDMs were stimulated for 6 hr with live GBS: NEM 316 (MOI 7:1), NEM Δ_cylE_ (MOI 7:1), NEM Δ_cfb_ (MOI 6:1), and NEM Δ_cylE_Δ_cfb_ (MOI 7:1). Levels of mRNA for IFN-β were determined as mentioned in Figure 1. *, P < 0.05, Student’s t test.
Figure 5. Degradation of the Bacteria Is Required for the Release of the IFN-Inducing Ligand
(A and B) WT BMDMs were pretreated for 30 min with bafilomycin A1 (200 nM) (A) or chymostatin (100 μM) (B) before being infected with live GBS (MOI 6:1) for 6 hr or Sendai virus (150 HAU/ml) for 4 hr. The amount of IFN-β mRNA was determined by Q-PCR. *, P < 0.05, Student’s t test.
Figure 6. TBK1 and IRF3 Are Critical Components of the Pathway Activated by Live GBS
(A and B) TNFR1−/−- and TNFR1/TBK1- deficient BMDMs (A) and C57BL/6 and IRF3−/− BMDMs (B) were stimulated with live GBS (MOI 6:1) for 6 hr or Sendai virus (150 HAU/ml) for 4 hr, and IFN-β mRNA was measured as mentioned in Figure 1. In (B), phosphorylation of the transcription factor STAT2 was examined in WT and IRF3-deficient macrophages infected with live GBS. (C and D) In (C), C57BL/6 and MAVS-deficient BMDMs were infected with Sendai virus or GBS as mentioned above, and IFN-β was measured by Q-PCR. In (D), C57BL/6 BMDMs were infected with a retroviral vector encoding shRNA for DAI and DAI levels measured by Q-PCR before and after IFN-β treatment (250 U/ml) (left). On the right, cells infected with DAI shRNA under the same conditions were exposed to PBS or live GBS (MOI 1:6) for 6 hr, and IFN-β levels were measured by Q-PCR. *, P < 0.05, Student’s t test.
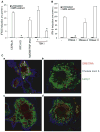
Figure 7. Induction of Type I IFNs by GBS DNA Has the Same Pathway Requirements as Induction by Live GBS
(A) GBS extract was transfected with lipofectamine into WT, IRF3-deficient, or TBK1-deficient BMDMs. *, P < 0.05, Student’s t test. (B) GBS extract was pretreated with vehicle, DNase I, RNase A, or RNase H and transfected into the cytosol of WT BMDMs, and IFN-β measured by Q-PCR. *, P < 0.05, Student’s t test. (C) Confocal microscopy of WT macrophages fed DNA-tagged live GBS (MOI 20:1) for 2 hr. GBS DNA was stained with syto60 dye (red), the phagosomal membrane with Lamp1 (green), and the plasma membrane with Cholera toxin b (blue). Untreated macrophages were fed WT (a) or hemolysin-deficient (b) NEM strain. Macrophages were treated with bafilomycin A1 (200 nM) (c) or chymostatin (100 μM) (d). The red arrows show GBS DNA located in a Lamp1-stained compartment, whereas white arrows highlight GBS DNA located outside of this compartment.
Similar articles
- cGAS-STING-TBK1-IRF3/7 induced interferon-β contributes to the clearing of non tuberculous mycobacterial infection in mice.
Ruangkiattikul N, Nerlich A, Abdissa K, Lienenklaus S, Suwandi A, Janze N, Laarmann K, Spanier J, Kalinke U, Weiss S, Goethe R. Ruangkiattikul N, et al. Virulence. 2017 Oct 3;8(7):1303-1315. doi: 10.1080/21505594.2017.1321191. Epub 2017 Apr 19. Virulence. 2017. PMID: 28422568 Free PMC article. - Critical role of TRAF3 in the Toll-like receptor-dependent and -independent antiviral response.
Oganesyan G, Saha SK, Guo B, He JQ, Shahangian A, Zarnegar B, Perry A, Cheng G. Oganesyan G, et al. Nature. 2006 Jan 12;439(7073):208-11. doi: 10.1038/nature04374. Epub 2005 Nov 23. Nature. 2006. PMID: 16306936 - Mechanisms and pathways of innate immune activation and regulation in health and cancer.
Cui J, Chen Y, Wang HY, Wang RF. Cui J, et al. Hum Vaccin Immunother. 2014;10(11):3270-85. doi: 10.4161/21645515.2014.979640. Hum Vaccin Immunother. 2014. PMID: 25625930 Free PMC article. Review. - Scaling of immune responses against intracellular bacterial infection.
Abdullah Z, Knolle PA. Abdullah Z, et al. EMBO J. 2014 Oct 16;33(20):2283-94. doi: 10.15252/embj.201489055. Epub 2014 Sep 15. EMBO J. 2014. PMID: 25225613 Free PMC article. Review.
Cited by
- Intracellular pathogen detection by RIG-I-like receptors.
Dixit E, Kagan JC. Dixit E, et al. Adv Immunol. 2013;117:99-125. doi: 10.1016/B978-0-12-410524-9.00004-9. Adv Immunol. 2013. PMID: 23611287 Free PMC article. Review. - A host type I interferon response is induced by cytosolic sensing of the bacterial second messenger cyclic-di-GMP.
McWhirter SM, Barbalat R, Monroe KM, Fontana MF, Hyodo M, Joncker NT, Ishii KJ, Akira S, Colonna M, Chen ZJ, Fitzgerald KA, Hayakawa Y, Vance RE. McWhirter SM, et al. J Exp Med. 2009 Aug 31;206(9):1899-911. doi: 10.1084/jem.20082874. Epub 2009 Aug 3. J Exp Med. 2009. PMID: 19652017 Free PMC article. - Interaction of Streptococcus agalactiae and Cellular Innate Immunity in Colonization and Disease.
Landwehr-Kenzel S, Henneke P. Landwehr-Kenzel S, et al. Front Immunol. 2014 Oct 29;5:519. doi: 10.3389/fimmu.2014.00519. eCollection 2014. Front Immunol. 2014. PMID: 25400631 Free PMC article. Review. - Suppression of local type I interferon by gut microbiota-derived butyrate impairs antitumor effects of ionizing radiation.
Yang K, Hou Y, Zhang Y, Liang H, Sharma A, Zheng W, Wang L, Torres R, Tatebe K, Chmura SJ, Pitroda SP, Gilbert JA, Fu YX, Weichselbaum RR. Yang K, et al. J Exp Med. 2021 Mar 1;218(3):e20201915. doi: 10.1084/jem.20201915. J Exp Med. 2021. PMID: 33496784 Free PMC article. - Immune sensing of DNA.
Paludan SR, Bowie AG. Paludan SR, et al. Immunity. 2013 May 23;38(5):870-80. doi: 10.1016/j.immuni.2013.05.004. Immunity. 2013. PMID: 23706668 Free PMC article. Review.
References
- Akira S, Sato S. Toll-like receptors and their signaling mechanisms. Scand J Infect Dis. 2003;35:555–562. - PubMed
- Baron MJ, Bolduc GR, Goldberg MB, Auperin TC, Madoff LC. Alpha C protein of group B Streptococcus binds host cell surface glycosaminoglycan and enters cells by an actin-dependent mechanism. J Biol Chem. 2004;279:24714–24723. - PubMed
- Berche P, Gaillard JL, Richard S. Invasiveness and intracellular growth of Listeria monocytogenes. Infection. 1988;16(Suppl 2):S145–S148. - PubMed
- Chamaillard M, Hashimoto M, Horie Y, Masumoto J, Qiu S, Saab L, Ogura Y, Kawasaki A, Fukase K, Kusumoto S, et al. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat Immunol. 2003;4:702–707. - PubMed
- Cieslewicz MJ, Kasper DL, Wang Y, Wessels MR. Functional analysis in type Ia group B streptococcus of a cluster of genes involved in extracellular polysaccharide production by diverse species of streptococci. J Biol Chem. 2001;276:139–146. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 AI007061/AI/NIAID NIH HHS/United States
- R01 AI067497/AI/NIAID NIH HHS/United States
- 2AI052455/AI/NIAID NIH HHS/United States
- R01 AI052455-04/AI/NIAID NIH HHS/United States
- R37 AI067497/AI/NIAID NIH HHS/United States
- R56 AI052455/AI/NIAID NIH HHS/United States
- R01 AI052455/AI/NIAID NIH HHS/United States
- R56 AI067497/AI/NIAID NIH HHS/United States
- R01 AI052455-03/AI/NIAID NIH HHS/United States
- AI067497/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous