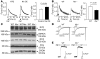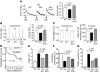Endoplasmic reticulum-mitochondria crosstalk in NIX-mediated murine cell death - PubMed (original) (raw)
. 2009 Jan;119(1):203-12.
doi: 10.1172/JCI36445. Epub 2008 Dec 8.
Affiliations
- PMID: 19065046
- PMCID: PMC2613462
- DOI: 10.1172/JCI36445
Endoplasmic reticulum-mitochondria crosstalk in NIX-mediated murine cell death
Abhinav Diwan et al. J Clin Invest. 2009 Jan.
Abstract
Transcriptional upregulation of the proapoptotic BCL2 family protein NIX limits red blood cell formation and can cause heart failure by inducing cell death, but the requisite molecular events are poorly defined. Here, we show complementary mechanisms for NIX-mediated cell death involving direct and ER/sarcoplasmic reticulum-mediated (ER/SR-mediated) mitochondria disruption. Endogenous cardiac NIX and recombinant NIX localize both to the mitochondria and to the ER/SR. In genetic mouse models, cardiomyocyte ER/SR calcium stores are proportional to the level of expressed NIX. Whereas Nix ablation was protective in a mouse model of apoptotic cardiomyopathy, genetic correction of the decreased SR calcium content of Nix-null mice restored sensitivity to cell death and reestablished cardiomyopathy. Nix mutants specific to ER/SR or mitochondria activated caspases and were equally lethal, but only ER/SR-Nix caused loss of the mitochondrial membrane potential. These results establish a new function for NIX as an integrator of transcriptional and calcium-mediated signals for programmed cell death.
Figures
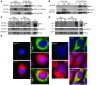
Figure 1. Nix localizes to mitochondria and ER and cofractionates with mitochondrial and ER/SR proteins.
(A) HEK293 cells were transfected with FLAG-Nix or β-gal control; fractionated into 10,000 g pellet (10p), 100,000 g pellet (100p), and 100,000 g supernatant (S); and immunoblotted with anti-FLAG, calnexin (Cal), or COX IV antibodies. (B) Neonatal rat ventricular myocytes were infected with adenoviruses encoding FLAG-Nix or β-gal control and processed as in A. E, empty lane. (C and D) Hearts from mice subjected to 1 week of pressure overload (TAC) and nonoperated controls (Non) were fractionated into a 10,000 g pellet and a 100,000 g pellet. The 100,000 g pellet was separated on a discontinuous sucrose gradient to yield the SR-rich fraction (SR, see Methods). 50 μg (C) and 20 μg (D) of the indicated fractions were separated by SDS-PAGE and immunoblotted with anti-Bnip3L (Nix), calnexin, or COX IV antibodies. Positive control (+C) (10 μg) was cellular extract from FLAG-Nix transfected HEK293 cells, showing multiple bands corresponding to Nix homodimers and heterodimers. (E) FLAG epitope–tagged Nix or β-gal control (both green) were transiently expressed in HEK293 cells and analyzed by fluorescence microscopy for colocalization with mitochondrial MitoFluor Red 589 (Mito) or ER calnexin (both red). Nuclei are blue (DAPI). Overlay for Nix is at bottom. Original magnification, ×1,000. Scale bar: 10 μm (shown for comparison).
Figure 2. Nix regulates ER and SR calcium stores.
(A) Ventricular cardiac myocytes isolated from nontransgenic (NTG) or conditional Nix-overexpressing (Nix OE) mouse hearts were loaded with Fura-2 AM and analyzed for caffeine-stimulated [Ca2+]i by monitoring the 510 nm emission during rapidly alternating excitation at 340 and 380 nm. Data are reported as the 340:380 nm emission ratio. A representative pair of tracings is shown (left). Group data (right) represent mean ± SEM of 24 NTG and 42 Nix OE cardiac myocytes from 3 pairs of hearts. Caf, caffeine. (B) Ventricular cardiac myocytes isolated from WT or Nix-knockout (Nix–/–) mouse hearts were loaded with Fura-2 AM and analyzed for caffeine-stimulated [Ca2+]i as above. A representative pair of tracings is shown (left). Group data (right) represent mean ± SEM of 25 WT and 50 Nix–/– cardiac myocytes from 5 pairs of hearts. (C) Crude cardiac extracts from _Nix_-null (Nix–/–) and WT hearts were subjected to immunoblotting for RYR, SERCA, NCX, PLN, and CSQN (50 μg protein/lane). (D) Representative peak ICa traces recorded from a holding potential of –50 mV to the indicated test potentials in patch-clamped isolated _Nix_-null and WT cardiac myocytes. (E) Representative traces of Na+/Ca2+ exchange current induced by a rapid solution change from 150 mM Na+ to 150 mM Li+ (indicated above) at a holding potential of –40 mV, recorded from _Nix_-null and WT cardiac myocytes.
Figure 3. In vivo restoration of SR calcium stores in _Nix_-knockout cardiac myocytes by SERCA disinhibition reverses the _Nix_-null rescue of Gq peripartum cardiomyopathy.
(A) Ventricular cardiac myocytes isolated from WT, Nix-knockout (Nix–/–), or Nix/PLN-DKO (Nix–/–PLN–/–) mouse hearts were loaded with Fura-2 AM and analyzed for caffeine-stimulated [Ca2+]i. A representative set of tracings is shown (left). Group data (right) represent mean ± SEM of 35 WT, 28 Nix–/–, and 38 Nix–/–PLN–/– cardiac myocytes from n = 3 to 4 hearts each. (B) Pacing-stimulated [Ca2+]i in ventricular myocytes from the same groups. Representative tracings are shown (left) and group data represented as mean ± SEM (right). (C) Contraction of paced ventricular myocytes from the same groups. Representative contraction tracings are shown as the absolute change in cell length over time (left). Quantitative analysis of the peak rate of change of cell shortening are represented as mean ± SEM (right). Horizontal bars indicate time. (D) Kaplan-Meier analysis of mouse survival in the peripartum period. Daily survival of Gq (n = 36), Gq Nix–/– (n = 30), and Gq _Nix–/–_PLN–DKO (Gq DKO, n = 19) dams was assessed after giving birth. log-rank statistic was employed to detect statistical significance. *P = 0.017 versus Gq by post-hoc test (Holms-Sidak). (E–G) Comparative analysis of left ventricular dilation (E, measured as the ratio of ventricular radius [r] to wall thickness [h]), contraction (F, measured as echocardiographic percentage of fractional shortening), and apoptosis (G, measured as the percentage of TUNEL-positive cardiac myocytes) for the same study groups (n = 6–13/group). WT is shown for comparison with normal. Statistical significance was determined by 1-way ANOVA and Tukey’s post-hoc testing.
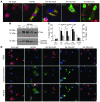
Figure 4. Creation of mitochondria- and ER-specific Nix mutants.
(A) Calcium-induced swelling of purified WT (triangles) and _Nix_-knockout (diamonds) liver mitochondria. Calcium (250 μM) was added (arrow) and the decrease in absorbance of 540 nm light assessed over time. Each curve represents the mean of 2 separate experiments. (B) Schematic depiction of mutation strategy for organelle-specific Nix. TM, putative transmembrane domain. (C and D) FLAG epitope–tagged WT Nix (Nix-WT), Nix-ActA, Nix-cb5, or truncated Nix (Nix-trunc) were transiently expressed in HEK293 cells, fractionated into 10,000 g pellet, 100,000 g pellet, and 100,000 g supernatant, and immunoblotted with anti-FLAG, calnexin, or COX IV antibodies (25 μg protein/lane). (D) Transiently transfected WT Nix, Nix-ActA, Nix-cb5, or truncated Nix (all green) as in C were analyzed by fluorescence microscopy for colocalization with MitoFluor Red 589 or ER calnexin (both red). Nuclei are blue (DAPI). Overlays are shown at bottom. Original magnification, ×1,000. Scale bar: 10 μm (shown for comparison).
Figure 5. ER- and mitochondria-targeted Nix are equally effective in killing cultured cardiac myocytes but utilize different mediators.
(A) Cultured neonatal rat cardiac myocytes were infected with adenoviruses encoding WT Nix or 1 of the 3 Nix mutants and subjected to fluorescence microscopy for subcellular localization. Shown are overlay images with FLAG-Nix (green) and MitoFluor Red 589 (red). Original magnification, ×1,000. Scale bar: 10 μm (shown for comparison). (B) Cultured neonatal rat cardiac myocytes were infected with adenoviruses encoding WT Nix or 1 of the 3 Nix mutants, and Nix expression was analyzed by immunoblotting for FLAG epitope and GAPDH (loading control; 25 μg/lane). Arrows indicate Nix mutants with varying molecular weights. (C) Quantitative analysis of cardiomyocyte death (left y axis, white bars) and TUNEL positivity in the absence (right y axis, black bars) and presence (right y axis, gray bars) of 25 μM BAPTA-AM induced by subcellular targeting of Nix. Means ± SEM of 4 independent experiments for death and 8 (–BAPTA) and 5 for TUNEL (+BAPTA) are shown. (D) Confocal microscopy of TMRE (red) and fluorescent caspase substrate (rhodamine 100 bis-
l
-aspartic acid amide; green) in cultured neonatal rat cardiac myocytes infected with adenoviruses encoding WT Nix or 1 of the 3 Nix mutants. Original magnification, ×400. Nuclei are blue (Hoechst 33342). Scale bar: 20 μm (shown for comparison).
Figure 6. Mechanisms of Nix-induced cell death.
Transcriptionally induced NIX is targeted to the mitochondria and ER/SR and activates programmed cell death in cells via a canonical mitochondrial pathway and what we believe is a novel ER/SR pathway. Mitochondria-targeted Nix causes mitochondrial outer membrane permeabilization, likely in coordination with Bax and Bak, leading to cytochrome c release, apoptosome formation, caspase 3 activation and apoptotic cell death. ER/SR-targeted Nix contributes to ER/SR calcium overload. This sensitizes cells to environmental stimuli, resulting in local calcium release at junctional “hot spots” with mitochondria. Released calcium is taken up by a mitochondrial uniporter, causing intramitochondrial calcium overload that triggers mitochondrial permeability pore formation with loss of mitochondrial potential, mitochondrial swelling, and release of essential mitochondrial proteins, preventing ATP generation with resultant bioenergetic failure. This is rapidly followed by failure of energy-dependent, plasma membrane–localized ion transporters, causing intracellular ion overload, cellular swelling and rupture, and necrotic cell death. MPTP, mitochondrial permeability transition pore.
Similar articles
- Dual autonomous mitochondrial cell death pathways are activated by Nix/BNip3L and induce cardiomyopathy.
Chen Y, Lewis W, Diwan A, Cheng EH, Matkovich SJ, Dorn GW 2nd. Chen Y, et al. Proc Natl Acad Sci U S A. 2010 May 18;107(20):9035-42. doi: 10.1073/pnas.0914013107. Epub 2010 Apr 23. Proc Natl Acad Sci U S A. 2010. PMID: 20418503 Free PMC article. - Role of the calcium-sensing receptor in cardiomyocyte apoptosis via the sarcoplasmic reticulum and mitochondrial death pathway in cardiac hypertrophy and heart failure.
Lu FH, Fu SB, Leng X, Zhang X, Dong S, Zhao YJ, Ren H, Li H, Zhong X, Xu CQ, Zhang WH. Lu FH, et al. Cell Physiol Biochem. 2013;31(4-5):728-43. doi: 10.1159/000350091. Epub 2013 May 23. Cell Physiol Biochem. 2013. PMID: 23711498 - Binding of FUN14 Domain Containing 1 With Inositol 1,4,5-Trisphosphate Receptor in Mitochondria-Associated Endoplasmic Reticulum Membranes Maintains Mitochondrial Dynamics and Function in Hearts in Vivo.
Wu S, Lu Q, Wang Q, Ding Y, Ma Z, Mao X, Huang K, Xie Z, Zou MH. Wu S, et al. Circulation. 2017 Dec 5;136(23):2248-2266. doi: 10.1161/CIRCULATIONAHA.117.030235. Epub 2017 Sep 23. Circulation. 2017. PMID: 28942427 Free PMC article. - SR/ER-mitochondrial local communication: calcium and ROS.
Csordás G, Hajnóczky G. Csordás G, et al. Biochim Biophys Acta. 2009 Nov;1787(11):1352-62. doi: 10.1016/j.bbabio.2009.06.004. Epub 2009 Jun 13. Biochim Biophys Acta. 2009. PMID: 19527680 Free PMC article. Review. - Functional implications of mitofusin 2-mediated mitochondrial-SR tethering.
Dorn GW 2nd, Song M, Walsh K. Dorn GW 2nd, et al. J Mol Cell Cardiol. 2015 Jan;78:123-8. doi: 10.1016/j.yjmcc.2014.09.015. Epub 2014 Sep 22. J Mol Cell Cardiol. 2015. PMID: 25252175 Free PMC article. Review.
Cited by
- Cell death in the myocardium: my heart won't go on.
Orogo AM, Gustafsson ÅB. Orogo AM, et al. IUBMB Life. 2013 Aug;65(8):651-6. doi: 10.1002/iub.1180. Epub 2013 Jul 3. IUBMB Life. 2013. PMID: 23824949 Free PMC article. Review. - Hypoxia regulates the degradation of non-nuclear organelles during lens differentiation through activation of HIF1a.
Brennan L, Disatham J, Kantorow M. Brennan L, et al. Exp Eye Res. 2020 Sep;198:108129. doi: 10.1016/j.exer.2020.108129. Epub 2020 Jul 3. Exp Eye Res. 2020. PMID: 32628953 Free PMC article. - Mitochondria signaling pathways in allergic asthma.
Qian L, Mehrabi Nasab E, Athari SM, Athari SS. Qian L, et al. J Investig Med. 2022 Apr;70(4):863-882. doi: 10.1136/jim-2021-002098. Epub 2022 Feb 15. J Investig Med. 2022. PMID: 35168999 Free PMC article. Review. - Mechanisms of non-apoptotic programmed cell death in diabetes and heart failure.
Dorn GW 2nd. Dorn GW 2nd. Cell Cycle. 2010 Sep 1;9(17):3442-8. doi: 10.4161/cc.9.17.12944. Epub 2010 Sep 7. Cell Cycle. 2010. PMID: 20814234 Free PMC article. Review. - Neurohormonal connections with mitochondria in cardiomyopathy and other diseases.
Dorn GW 2nd. Dorn GW 2nd. Am J Physiol Cell Physiol. 2022 Aug 1;323(2):C461-C477. doi: 10.1152/ajpcell.00167.2022. Epub 2022 Jun 27. Am J Physiol Cell Physiol. 2022. PMID: 35759434 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- HL077101/HL/NHLBI NIH HHS/United States
- R01 HL080008/HL/NHLBI NIH HHS/United States
- R01 HL059888/HL/NHLBI NIH HHS/United States
- HL080008/HL/NHLBI NIH HHS/United States
- HL059888/HL/NHLBI NIH HHS/United States
- P50 HL077101/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials