FAT/CD36 expression is not ablated in spontaneously hypertensive rats - PubMed (original) (raw)
FAT/CD36 expression is not ablated in spontaneously hypertensive rats
Arend Bonen et al. J Lipid Res. 2009 Apr.
Abstract
There is doubt whether spontaneously hypertensive rats (SHR; North American strain) are null for fatty acid translocase (FAT/CD36). Therefore, we examined whether FAT/CD36 is expressed in heart, muscle, liver and adipose tissue in SHR. Insulin resistance was present in SHR skeletal muscle. We confirmed that SHR expressed aberrant FAT mRNAs in key metabolic tissues; namely, the major 2.9 kb transcript was not expressed, but 3.8 and 5.4 kb transcripts were present. Despite this, FAT/CD36 protein was expressed in all tissues, although there were tissue-specific reductions in FAT/CD36 protein expression and plasmalemmal content, ranging from 26-85%. Fatty acid transport was reduced in adipose tissue (-50%) and was increased in liver (+47%). Normal rates of fatty acid transport occurred in heart and muscle, possibly due to compensatory upregulation of plasmalemmal fatty acid binding protein (FABPpm) in red (+123%) and white muscle (+110%). In conclusion, SHRs (North American strain) are not a natural FAT/CD36 null model, the North American strain of SHR express FAT/CD36, albeit at reduced levels.
Figures
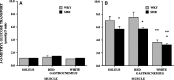
Fig. 1.
Basal (A) and insulin stimulated glucose transport (B) in hindlimb perfused muscles in WKY and spontaneously hypertensive rats (SHR). Hindlimb perfusion was performed using controlled flow rates and radiolabeled 3-O-methylglucose with (20 mU/ml) or without insulin (basal) as described in the Methods. Data are mean ± SEM, N = 4 for each treatment * P < 0.05, SHR vs. WKY ** P < 0.05, white muscle vs. red muscle.
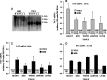
Fig. 2.
FAT mRNA blot in heart (H), red (R) and white (W) muscle, adipose tissue (A), and liver (L) of WKY and SHR (A) and the quantification of 2.9 kb (B), 3.8 kb (C), and 5.4 kb (D) FAT transcripts in WKY and SHR. Data were quantified by determining the density of each blot, which was normalized for loading using 28S RNA. Data are mean ± SEM, N = 3–4 for each tissue examined in each group of animals SHR. ND, not detected.
Fig. 3.
Protein expression of fatty acid translocase (FAT/CD36) in heart, red and white skeletal muscle, adipose tissue, and liver in WKY and SHR. Data are mean ± SEM, N = 5–8 for each tissue examined in each group of animals. R, red muscle; W, white muscle; H, heart; A, adipose tissue; L, liver FAT/CD36 was detected at 88 kDa. Data were quantified by determining the density of each blot and normalized using Ponceau staining. Ponceau stains are shown below Western blot for FAT/CD36 * P < 0.05, SHR vs. WKY. Inset: FAT/CD36 protein expression in tissue homogenates using a commercially available antibody.
Fig. 4.
Plasma membrane FAT/CD36 in heart, red and white skeletal muscle, adipose tissue, and liver in WKY and SHR. Data are mean ± SEM, N = 4–8 for each tissue examined in each group of animals. R, Red muscle; W, white muscle; H, heart; A, adipose tissue; L, liver FAT/CD36 was detected at 88 kDa. Data were quantified by determining the density of each blot and normalized using Ponceau staining. Ponceau stains are shown below Western blot for FAT/CD36 * P < 0.05, SHR vs. WKY. Inset: plasma membrane FAT/CD36 protein using a commercially available antibody.
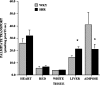
Fig. 5.
Rate of palmitate transport into giant vesicles prepared from heart, red and white skeletal muscle, liver, and adipose tissue in WKY and SHR. Data are mean ± SEM, N = 6–8 for each tissue examined in each group of animals * P < 0.05, SHR vs. WKY.
Similar articles
- Defective fatty acid uptake in the spontaneously hypertensive rat is a primary determinant of altered glucose metabolism, hyperinsulinemia, and myocardial hypertrophy.
Hajri T, Ibrahimi A, Coburn CT, Knapp FF Jr, Kurtz T, Pravenec M, Abumrad NA. Hajri T, et al. J Biol Chem. 2001 Jun 29;276(26):23661-6. doi: 10.1074/jbc.M100942200. Epub 2001 Apr 25. J Biol Chem. 2001. PMID: 11323420 - Cardiac and skeletal muscle fatty acid transport and transporters and triacylglycerol and fatty acid oxidation in lean and Zucker diabetic fatty rats.
Bonen A, Holloway GP, Tandon NN, Han XX, McFarlan J, Glatz JF, Luiken JJ. Bonen A, et al. Am J Physiol Regul Integr Comp Physiol. 2009 Oct;297(4):R1202-12. doi: 10.1152/ajpregu.90820.2008. Epub 2009 Aug 12. Am J Physiol Regul Integr Comp Physiol. 2009. PMID: 19675275 - Metabolic challenges reveal impaired fatty acid metabolism and translocation of FAT/CD36 but not FABPpm in obese Zucker rat muscle.
Han XX, Chabowski A, Tandon NN, Calles-Escandon J, Glatz JF, Luiken JJ, Bonen A. Han XX, et al. Am J Physiol Endocrinol Metab. 2007 Aug;293(2):E566-75. doi: 10.1152/ajpendo.00106.2007. Epub 2007 May 22. Am J Physiol Endocrinol Metab. 2007. PMID: 17519284 - Evidence for concerted action of FAT/CD36 and FABPpm to increase fatty acid transport across the plasma membrane.
Chabowski A, Górski J, Luiken JJ, Glatz JF, Bonen A. Chabowski A, et al. Prostaglandins Leukot Essent Fatty Acids. 2007 Nov-Dec;77(5-6):345-53. doi: 10.1016/j.plefa.2007.10.017. Prostaglandins Leukot Essent Fatty Acids. 2007. PMID: 18240411 Review. - Regulation of fatty acid transport by fatty acid translocase/CD36.
Bonen A, Campbell SE, Benton CR, Chabowski A, Coort SL, Han XX, Koonen DP, Glatz JF, Luiken JJ. Bonen A, et al. Proc Nutr Soc. 2004 May;63(2):245-9. doi: 10.1079/PNS2004331. Proc Nutr Soc. 2004. PMID: 15294038 Review.
Cited by
- CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior.
Silverstein RL, Febbraio M. Silverstein RL, et al. Sci Signal. 2009 May 26;2(72):re3. doi: 10.1126/scisignal.272re3. Sci Signal. 2009. PMID: 19471024 Free PMC article. Review. - Metabolic Changes in Spontaneously Hypertensive Rat Hearts Precede Cardiac Dysfunction and Left Ventricular Hypertrophy.
Li J, Kemp BA, Howell NL, Massey J, Mińczuk K, Huang Q, Chordia MD, Roy RJ, Patrie JT, Davogustto GE, Kramer CM, Epstein FH, Carey RM, Taegtmeyer H, Keller SR, Kundu BK. Li J, et al. J Am Heart Assoc. 2019 Feb 19;8(4):e010926. doi: 10.1161/JAHA.118.010926. J Am Heart Assoc. 2019. PMID: 30764689 Free PMC article. - The anti-TNF-α antibody infliximab inhibits the expression of fat-transporter-protein FAT/CD36 in a selective hepatic-radiation mouse model.
Martius G, Cameron S, Rave-Fränk M, Hess CF, Wolff HA, Malik IA. Martius G, et al. Int J Mol Sci. 2015 Mar 2;16(3):4682-97. doi: 10.3390/ijms16034682. Int J Mol Sci. 2015. PMID: 25739082 Free PMC article. - A low-carbohydrate/high-fat diet reduces blood pressure in spontaneously hypertensive rats without deleterious changes in insulin resistance.
Bosse JD, Lin HY, Sloan C, Zhang QJ, Abel ED, Pereira TJ, Dolinsky VW, Symons JD, Jalili T. Bosse JD, et al. Am J Physiol Heart Circ Physiol. 2013 Jun 15;304(12):H1733-42. doi: 10.1152/ajpheart.00631.2012. Epub 2013 Apr 19. Am J Physiol Heart Circ Physiol. 2013. PMID: 23604708 Free PMC article. - Transgenic rescue of defective Cd36 enhances myocardial adenylyl cyclase signaling in spontaneously hypertensive rats.
Klevstig M, Manakov D, Kasparova D, Brabcova I, Papousek F, Zurmanova J, Zidek V, Silhavy J, Neckar J, Pravenec M, Kolar F, Novakova O, Novotny J. Klevstig M, et al. Pflugers Arch. 2013 Oct;465(10):1477-86. doi: 10.1007/s00424-013-1281-5. Epub 2013 May 1. Pflugers Arch. 2013. PMID: 23636771
References
- Bonen A., A. Chabowski, J. J. F. P. Luiken, and J. F. C. Glatz. 2007. Is membrane transport of FFA mediated by lipid, protein, or both? Mechanisms and regulation of protein-mediated cellular fatty acid uptake: molecular, biochemical, and physiological evidence. Physiology (Bethesda). 22 15–29. - PubMed
- Kampf J. P., and A. M. Kleinfeld. 2007. Is membrane transport of FFA mediated by lipid, protein, or both? An unknown protein mediates free fatty acid transport across the adipocyte plasma membrane. Physiology (Bethesda). 22 7–14. - PubMed
- Kampf J. P., A. M. Kleinfeld, A. Bonen, A. Chabowski, J. J. F. P. Luiken, and J. F. C. Glatz. 2007. Is membrane transport of FFA mediated by lipid, protein, or both?: Points of agreement. Physiology (Bethesda). 22 29. - PubMed
- Stahl A. 2004. A current review of fatty acid transport proteins (SLC27). Pflugers Arch. 447 722–727. - PubMed
- Febbraio M., N. A. Abumrad, D. P. Hajjar, K. Sharma, W. Cheng, S. Frieda, A. Pearce, and R. L. Silverstein. 1999. A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. J. Biol. Chem. 274 19055–19062. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical