COPI complex is a regulator of lipid homeostasis - PubMed (original) (raw)
COPI complex is a regulator of lipid homeostasis
Mathias Beller et al. PLoS Biol. 2008.
Abstract
Lipid droplets are ubiquitous triglyceride and sterol ester storage organelles required for energy storage homeostasis and biosynthesis. Although little is known about lipid droplet formation and regulation, it is clear that members of the PAT (perilipin, adipocyte differentiation related protein, tail interacting protein of 47 kDa) protein family coat the droplet surface and mediate interactions with lipases that remobilize the stored lipids. We identified key Drosophila candidate genes for lipid droplet regulation by RNA interference (RNAi) screening with an image segmentation-based optical read-out system, and show that these regulatory functions are conserved in the mouse. Those include the vesicle-mediated Coat Protein Complex I (COPI) transport complex, which is required for limiting lipid storage. We found that COPI components regulate the PAT protein composition at the lipid droplet surface, and promote the association of adipocyte triglyceride lipase (ATGL) with the lipid droplet surface to mediate lipolysis. Two compounds known to inhibit COPI function, Exo1 and Brefeldin A, phenocopy COPI knockdowns. Furthermore, RNAi inhibition of ATGL and simultaneous drug treatment indicate that COPI and ATGL function in the same pathway. These data indicate that the COPI complex is an evolutionarily conserved regulator of lipid homeostasis, and highlight an interaction between vesicle transport systems and lipid droplets.
Conflict of interest statement
Competing interests. The authors have declared that no competing interests exist.
Figures
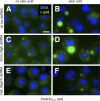
Figure 1. Drosophila Cells as a Model of Lipid Storage
(A–F) Drosophila Kc167 cells grown in standard medium (A, C, and E) or medium supplemented with 400 μM oleic acid complexed to 0.4% BSA (B, D, and F). Cells were treated with dsRNA targeting brummer (bmm; [C and D]) or midway (mdy; [E and F]). The corresponding mouse genes are Atgl and Dgat-1. Nuclei are shown in blue (stained with Hoechst 33342), and lipid droplets in green (stained with BODIPY493/503). Scale bar in (A) represents 10 μm.
Figure 2. RNAi-Mediated Genome-Wide Screening of Drosophila Cells for Lipid Storage Phenotypes
(A and B) Regions of original images from the screen of cells stained with DAPI (A) or the lipid droplet-specific dye BODIPY 493/503 (B). (C and D) Image segmentation results of the images shown in (A and B). Nuclei and lipid droplet boundaries are depicted. (E) Sample off-line lipid droplet quantification results by image segmentation. Cells were grown with or without oleic acid and treated with the indicated dsRNAs. Significant differences between dsRNA treated and untreated cells at p < 0.05 (two-sided _t_-test) are shown (indicated with an asterisk [*]). Error bars indicate standard deviation. (F) Rank-order plot of the normalized screen results where positive _B_-scores indicate overstorage and negative _B_-scores indicate understorage. (G) Performance of controls incorporated in the on-line screening plates. Black bars represent overstorage (_B_-score > 2.0), and light grey bars represent understorage phenotypes (_B_-score < −1.7).
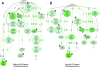
Figure 3. Independent, Genome-Wide RNAi Screens Identify Similar Sets of Genes Regulating Lipid Storage
GO-based functional classification (GO: biological process) of genes resulting in lipid storage phenotypes upon targeting by dsRNAs of the present (526 genes) as well as a concurrent study (227 genes; [52]). GO terms were organized into networks using the VLAD software (A and B). Node labels include the associated GO-term identifier and GO-term description as well as the associated _p_-value reflecting enrichment as compared to the whole-genome annotation reference set. The number of genes associated with the respective GO term included in the test set as well as the complete-genome annotation is also shown. A darker node color reflects lower _p_-values. Yellow stars indicate GO-terms shared by the two genome-wide RNAi screen datasets.
Figure 4. Enriched GO Terms among the Genes Promoting Lipid Storage
GO-based functional classification ([A] GO: molecular function; [B] GO: cellular component, and [C] GO: biological process) of genes resulting in lipid understorage phenotypes when targeted by dsRNAs (208 genes). For details, see main text and legend of Figure 3.
Figure 5. Enriched GO Terms among the Genes Promoting Lipid Utilization
GO-based functional classification ([A] GO: molecular function; [B] GO: cellular component, and [C] GO: biological process) of genes resulting in lipid overstorage phenotypes when targeted by dsRNAs (318 genes). For details, see main text and legend of Figure 3.
Figure 6. Evolutionary Conserved Lipid Droplet Regulators
Mouse AML12 cells store little lipid in the absence of exogenous NEFA (A), whereas addition of NEFA to the growth medium induces lipid droplet deposition (B). Knockdown effects on cellular lipid droplets of the mouse genes encoding Uqcrq (CG7580 in Drosophila), an electron transport chain member, and Smarca4 (Brahma in Drosophila), a chromatin-associated protein (C and D). Nuclei are shown in blue (stained with Hoechst 33342), lipid droplets in green (stained with BODIPY493/503). Scale bar in (A) represents 50 μm.
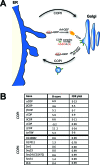
Figure 7. COPI Is a Regulator of Lipid Storage
(A) Schematic representation of the COPI trafficking pathway (center) directing traffic from the Golgi (right) to the ER (left). COPII members (not detailed) direct traffic from the ER to the Golgi. Members showing increased lipid storage following knockdown in the Drosophila primary screen are highlighted (red). (B) Primary screen results for COPI and COPII components are tabulated. The average _B_-score for the given gene (higher _B_-scores indicate more stored lipid, see Figure 2) as well as the average false discovery rate (FDR)-corrected _p_-value are given.
Figure 8. The COPI-Mediated Retrograde Trafficking Pathway Is a Negative Regulator of Lipid Storage
(A–F) Drosophila cells with or without oleic acid stained with BODIPY493/503 to detect lipid. Control cells not treated with dsRNA (A and D) or cells incubated with dsRNAs targeting Arf79F (an Arf1 homolog) (B and E) or α_Cop_ (C and F) are shown. (G) All COPI, and several COPII members as well as additional Arfs, were retested using independent dsRNAs and gave similar results. Results and number of dsRNAs present in the primary screen (including oleic acid) and retests are given. (H) Dose response of Drosophila S3 cells to Exo1 (structure inset) showing the %-activity derived either from the lipid specific signal (filled circles [•]) or the lipid/cell ratio (open circles [○]). Percent activity refers hereby to the changes of lipid storage relative to Triacsin C treatment, which decreases lipid storage by blocking TG synthesis (this is analogous to the mdy controls in RNAi experiments, see Material and Methods). Increased activity indicates increased lipid storage, which increased with concentration. Scale bar in (A) represents 10 μm.
Figure 9. Function of Selected Mouse Orthologs of Genes Showing Lipid Storage Phenotypes in Drosophila Cells
(A–F and M–R) AML12 or 3T3-L1 cells (G–L), with (B–F and H–R), or without (A and G) oleic acid and stained for nuclei (Hoechst 33342) and lipid (BODIPY493/503). Cells were transfected with ALLStars negative control siRNA (control) or the siRNAs targeting the indicated genes. Drosophila homologs are given (parentheses). Scale bar in (A) represents 50 μm.
Figure 10. NEFA Incorporation and NEFA Release Measured in AML12 Cells after RNAi-Mediated Gene Knockdown and Drug Treatment
(A) Relative activity [(experimental/ALLStars negative control (control)) − 1] in radiolabel assays for TG esterification (nM) (grey bar) and NEFA release (nM) (black) relative to total protein concentration in cells treated with siRNAs targeting the indicated transcripts. Significance at p < 0.01, impaired _t_-test, is shown (indicated by an asterisk [*]). Standard error is indicated by the bars. (B) Relative activity [(experimental/ALLStars negative control (control) and DMSO) − 1] in radiolabel assays for NEFA release (nM) relative to total protein concentration in cells treated with siRNAs targeting the indicated transcripts in the presence of DMSO only (open bar), BFA (5 μM) in DMSO (light-grey bar), or Exo1 (5μM) in DMSO (dark-grey bar). Significance at p < 0.01, impaired _t_-test, is shown (indicated by an asterisk [*]). Standard error is indicated by the bars.
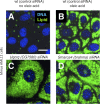
Figure 11. TIP47 Translocates to the Lipid Droplet Surface upon Down-Regulation of COPI
Mouse AML12 cells were treated with ALLStars negative control siRNA (control) (A) or siRNAs targeting γCOP (B) for 4 d, respectively. Cells were incubated with 400 μM oleic acid for 12 h prior to staining with antibodies detecting TIP47 (green channel) or ADRP (red channel). Images of the separate channels as well as a merged image are shown. (C–H) Merged channel images of similarly treated mouse AML12 cells. Targeted genes are indicated. Scale bar in (A) represents 50 μm.
Figure 12. Changes in the Abundance of Selected Lipid Droplet Proteome Members Following BFA Treatment
(A and B) Mouse AML12 cells were grown in the presence of NEFA (400 μM oleic acid) overnight and incubated in DMSO (A) or 5 μM BFA in DMSO (B) for 6 h, prior to staining with antibodies detecting TIP47 (green channel) or ADRP (red channel). Separate images for the different channels as well as a merged image are shown. (C) Western blots of lipid droplet protein preparations. Lipid droplets from AML12 cells grown in the presence of 400 μM oleic acid and treated with either DMSO (lanes one and three) or 5 μM BFA in DMSO (lanes two and four) were purified by sucrose gradient ultracentrifugation, and equal amounts of the associated proteins were subjected to western blot. Two separate experiment replicates are represented. Band density ratios (±BFA) are given. Scale bar in (A) represents 50 μm.
Figure 13. COPI Trafficking and Lipid Droplet Model
COPI and COPII trafficking systems shuttle proteins and lipids between ER and Golgi. Small TIP47-coated lipid droplets are born at the ER and reside in the cytosol. The bigger lipid droplets are coated by ADRP, and TIP47 is returned to the ER. ATGL can translocate between the cytosol and the lipid droplet surface, where it begins the conversion of TG to DG and eventually NEFA. Other TG lipases may also be present (indicated by the second arrow liberating TG from the hydrophobic lipid droplet core). Wild-type Golgi–ER transport function of COPI might be necessary for proper lipid droplet biogenesis at the ER. COPI could directly function at the lipid droplet surface, mediating a similar ER-targeted transport function as reported for the Golgi–ER COPI transport. COPI is a positive regulator of ATGL with a unresolved mechanism (indicated by the question mark) and a negative regulator of TIP47 lipid droplet localization. ADRP and TIP47 are negative regulators of ATGL activity at the lipid droplet surface.
Similar articles
- The contribution of the Drosophila model to lipid droplet research.
Kühnlein RP. Kühnlein RP. Prog Lipid Res. 2011 Oct;50(4):348-56. doi: 10.1016/j.plipres.2011.04.001. Epub 2011 May 17. Prog Lipid Res. 2011. PMID: 21620889 Review. - Arf1/COPI machinery acts directly on lipid droplets and enables their connection to the ER for protein targeting.
Wilfling F, Thiam AR, Olarte MJ, Wang J, Beck R, Gould TJ, Allgeyer ES, Pincet F, Bewersdorf J, Farese RV Jr, Walther TC. Wilfling F, et al. Elife. 2014;3:e01607. doi: 10.7554/eLife.01607. Epub 2014 Feb 4. Elife. 2014. PMID: 24497546 Free PMC article. - Opposite and redundant roles of the two Drosophila perilipins in lipid mobilization.
Bi J, Xiang Y, Chen H, Liu Z, Grönke S, Kühnlein RP, Huang X. Bi J, et al. J Cell Sci. 2012 Aug 1;125(Pt 15):3568-77. doi: 10.1242/jcs.101329. Epub 2012 Apr 14. J Cell Sci. 2012. PMID: 22505614 - Consequences of lipid droplet coat protein downregulation in liver cells: abnormal lipid droplet metabolism and induction of insulin resistance.
Bell M, Wang H, Chen H, McLenithan JC, Gong DW, Yang RZ, Yu D, Fried SK, Quon MJ, Londos C, Sztalryd C. Bell M, et al. Diabetes. 2008 Aug;57(8):2037-45. doi: 10.2337/db07-1383. Epub 2008 May 16. Diabetes. 2008. PMID: 18487449 Free PMC article. - Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis.
Brasaemle DL. Brasaemle DL. J Lipid Res. 2007 Dec;48(12):2547-59. doi: 10.1194/jlr.R700014-JLR200. Epub 2007 Sep 18. J Lipid Res. 2007. PMID: 17878492 Review.
Cited by
- Enterovirus Replication Organelles and Inhibitors of Their Formation.
Li X, Wang M, Cheng A, Wen X, Ou X, Mao S, Gao Q, Sun D, Jia R, Yang Q, Wu Y, Zhu D, Zhao X, Chen S, Liu M, Zhang S, Liu Y, Yu Y, Zhang L, Tian B, Pan L, Chen X. Li X, et al. Front Microbiol. 2020 Aug 20;11:1817. doi: 10.3389/fmicb.2020.01817. eCollection 2020. Front Microbiol. 2020. PMID: 32973693 Free PMC article. Review. - Transcriptional networks controlling the cell cycle.
Bonke M, Turunen M, Sokolova M, Vähärautio A, Kivioja T, Taipale M, Björklund M, Taipale J. Bonke M, et al. G3 (Bethesda). 2013 Jan;3(1):75-90. doi: 10.1534/g3.112.004283. Epub 2013 Jan 1. G3 (Bethesda). 2013. PMID: 23316440 Free PMC article. - The regulation of carnitine palmitoyltransferase 1 (CPT1) mRNA splicing by nutrient availability in Drosophila fat tissue.
Truong HG, Nagengast AA, DiAngelo JR. Truong HG, et al. Biochem Biophys Rep. 2024 Feb 16;38:101661. doi: 10.1016/j.bbrep.2024.101661. eCollection 2024 Jul. Biochem Biophys Rep. 2024. PMID: 38384389 Free PMC article. - Dengue Virus Uses a Non-Canonical Function of the Host GBF1-Arf-COPI System for Capsid Protein Accumulation on Lipid Droplets.
Iglesias NG, Mondotte JA, Byk LA, De Maio FA, Samsa MM, Alvarez C, Gamarnik AV. Iglesias NG, et al. Traffic. 2015 Sep;16(9):962-77. doi: 10.1111/tra.12305. Epub 2015 Jun 29. Traffic. 2015. PMID: 26031340 Free PMC article. - FAT SIGNALS--lipases and lipolysis in lipid metabolism and signaling.
Zechner R, Zimmermann R, Eichmann TO, Kohlwein SD, Haemmerle G, Lass A, Madeo F. Zechner R, et al. Cell Metab. 2012 Mar 7;15(3):279-91. doi: 10.1016/j.cmet.2011.12.018. Cell Metab. 2012. PMID: 22405066 Free PMC article. Review.
References
- Brasaemle DL. Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J Lipid Res. 2007;48:2547–2559. - PubMed
- Kuerschner L, Moessinger C, Thiele C. Imaging of lipid biosynthesis: how a neutral lipid enters lipid droplets. Traffic. 2008;9:338–352. - PubMed
- Bostrom P, Andersson L, Rutberg M, Perman J, Lidberg U, et al. SNARE proteins mediate fusion between cytosolic lipid droplets and are implicated in insulin sensitivity. Nat Cell Biol. 2007;9:1286–1293. - PubMed
- Beller M, Riedel D, Jansch L, Dieterich G, Wehland J, et al. Characterization of the Drosophila lipid droplet subproteome. Mol Cell Proteomics. 2006;5:1082–1094. - PubMed
- Liu P, Ying Y, Zhao Y, Mundy DI, Zhu M, et al. Chinese hamster ovary K2 cell lipid droplets appear to be metabolic organelles involved in membrane traffic. J Biol Chem. 2004;279:3787–3792. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM067761/GM/NIGMS NIH HHS/United States
- R01 DK075017-02S1/DK/NIDDK NIH HHS/United States
- R01 DK075017/DK/NIDDK NIH HHS/United States
- ZIA DK015600-14/ImNIH/Intramural NIH HHS/United States
- R01 DK 075017-01A2/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases