Consequences of motor copy number on the intracellular transport of kinesin-1-driven lipid droplets - PubMed (original) (raw)
Consequences of motor copy number on the intracellular transport of kinesin-1-driven lipid droplets
George T Shubeita et al. Cell. 2008.
Abstract
The microtubule motor kinesin-1 plays central roles in intracellular transport. It has been widely assumed that many cellular cargos are moved by multiple kinesins and that cargos with more motors move faster and for longer distances; concrete evidence, however, is sparse. Here we rigorously test these notions using lipid droplets in Drosophila embryos. We first employ antibody inhibition, genetics, biochemistry, and particle tracking to demonstrate that kinesin-1 mediates plus-end droplet motion. We then measure how variation in kinesin-1 expression affects the forces driving individual droplets and estimate the number of kinesins actively engaged per droplet. Unlike in vitro, increased motor number results in neither longer travel distances nor higher velocities. Our data suggest that cargos in vivo can simultaneously engage multiple kinesins and that transport properties are largely unaffected by variation in motor number. Apparently, higher-order regulatory mechanisms rather than motor number per se dominate cargo transport in vivo.
Figures
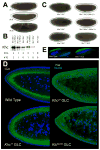
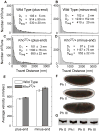
Figure 1. Net transport of lipid droplets requires Kinesin-1
(A) Lipid-droplet distribution in wild-type embryos, as revealed by transparency of the embryo periphery in transmitted light. During Phases I (top) and III (bottom), the periphery is opaque because lipid droplets are present throughout. In Phase II (middle), droplets move inward and as a result the periphery is transparent (“clearing”). (B) Khc levels in embryos of various genotypes. Top: full description of genotypes; bottom: summary how many copies of the endogenous Khc gene (#EG) and the Khc transgene P{Khc+} (#TG) are present. Proteins were extracted from embryos laid by mothers of the indicated genotypes, and Khc was detected by Western analysis. Coomassie Blue staining of membranes demonstrated equal protein loading across lanes (not shown). (C) Kinesin-1 function is required for clearing of the embryonic periphery in Phase II. When embryos either completely lack one of the Kinesin-1 subunits (Khc27 GLC and Klc8ex94 GLC) or contain only mutant Kinesin (Khc23 GLC and Khc17 GLC), they fail to undergo clearing during Phase II. Expression of wild-type Khc (from the P{Khc+} transgene) restores clearing in the absence of endogenous Khc (Khc27 GLCs) or when all endogenous Khc is mutant (embryos from mothers homozygous for Khc23, rescued to viability by P{Khc+}). (D) Kinesin-1 function is required for net inward transport of lipid droplets in Phase II. Embryos of various genotypes were stained for DNA (blue) and the droplet-marker Klar (green). In the wild type, Klar accumulates basally, away from the peripheral nuclei (blue) in Phase II, due to net plus-end droplet transport. When Kinesin-1 is impaired (Khc17 GLC), Klar is uniformly distributed in the peripheral cytoplasm below the nuclei. When either of the Kinesin-1 subunits is entirely absent (Khc27 or Klc8ex94 GLC), Klar is present not only throughout the peripheral cytoplasm, but also apical to nuclei and deep within the central yolk (where microtubules are not present). This severe mislocalization suggests that droplets are no longer attached to microtubules and diffuse throughout the embryo. (E) Droplet association of Klar does not require Kinesin-1. When embryos are centrifuged, the low-density lipid droplets accumulate in a distinct top layer, just above the nuclei (blue). Klar (green) is highly enriched in the droplet layer of both wild-type and Khc27 GLC embryos.
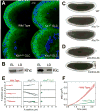
Figure 2. Kinesin-1 is directly responsible for droplet motion
(A) Microtubule tracks are present in embryos with disrupted Kinesin-1 function. Microtubules and nuclei were visualized by staining for β tubulin (green) and DNA (blue). Radially arranged microtubules are present in the embryo periphery of all genotypes shown, though their detailed arrangement is altered in the mutants: microtubules display less apical bundling (around the nuclei) and reach deeper into the embryos; sometimes regional patches are devoid of microtubules, possibly because nuclei failed to migrate to those regions earlier in development (not shown). (B) Both subunits of Kinesin-1 (Khc and Klc) are present on purified lipid droplets. Equal amounts of protein from total embryo lysate (EL) and biochemically purified lipid droplets (LD) were analyzed by Western blotting. (C) Khc alleles dominantly slow net droplet motion. Embryos from Khc17 or Khc23 heterozygous mothers (i.e. carrying both a mutant and a wild-type Khc allele) often display less efficient peripheral clearing in Phase II than embryos from wild-type (top) mothers. Embryos shown are at very similar developmental stages (see movie S7 for a time-lapse comparison). This clearing defect was variable in penetrance and severity; we estimate that at least half of embryos from either genotype were distinguishable from the wild type. (D) Acute inhibition of Kinesin-1 promotes net minus-end droplet transport. Wild-type mid-Phase II embryos were injected (on the right) with antibodies at concentrations of ∼2-3 μg/μl. Embryos injected with a function-blocking antibody against Khc (top) rapidly turned opaque near the injection site, indicating outward motion of lipid droplets. Injection of a control antibody (bottom) had no effect. (E) In the absence of Khc, droplet motion is severely impaired. Lipid droplets were tracked in Phase II embryos; three sample traces are shown per genotype. Wild-type (WT) and Khc27/+ embryos show similar traces with bidirectional transport; _Khc_27 GLCs display almost no directed motion. (F) Droplets in _Khc_27 GLC embryos exhibit minimal directed motion, as assessed by the Mean Squared Displacement as a function of time. The quadratic shape for wild type (WT) indicates directed transport; the reduced, linear MSD for the _Khc_27 GLC embryos is reminiscent of diffusion. Error bars are standard errors of the mean.
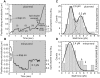
Figure 3. Quantifying droplet stall forces
(A) Example of stalling a plus-end moving droplet. The droplet initially stalled at 2.7 pN, the motor detached, and it was pulled to the center of the trap (i). The droplet was subsequently driven out of the trap with more force, did not stall and prematurely fell back to the trap center (ii). After another stall at 2.7 pN (iii), more motors engaged and generated enough force to pull it out of the influence of the trap (iv). (B) Example of stalling a minus-end moving droplet. (C) Stall-force distribution for plus-end moving droplets in the wild type. The histogram is fit (solid lines) with two Gaussian distributions centered at 2.6 and 5.2 pN. These values suggest that the peaks arise from lipid droplets moved by one and two motors, respectively. The fit did not include any restrictions on peak positions. Note that a number of droplets escape from the trap (rightmost bin), indicating the simultaneous action of even more motors. (D) Stall-force distribution for minus-end moving droplets in the wild type. The solid line is a Gaussian fit to the bins up to 4 pN. The bins above 4 pN (arrows) suggests a possible second peak (see text).
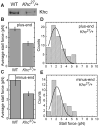
Figure 4. Droplets from _Khc27/_+ embryos have reduced Kinesin-1 levels and display lower stall forces
(A) Khc levels on embryonic lipid droplets are reduced in Khc heterozygotes (Khc27/+) compared to the wild type (WT). Equal amounts of total protein from purified lipid droplets were analyzed for Khc by Western blotting. This trend was reproduced in independent preparations; in some of them equal loading was additionally verified by probing for LSD2 (data not shown). (B, C) Average stall forces in Khc27/+ heterozygotes are reduced compared to the wild type, both for plus-end (B) and minus-end (C) moving droplets. (D, F) Stall-force distribution in Khc27/+ heterozygotes for plus-end (D) and minus-end (E) moving droplets. Both histograms show a single prominent peak whose position coincides with the 2.6± 0.5 pN peak in Fig. 3C, within the uncertainty estimated as half the bin size. (plus-end: 1.9 ± 0.5 pN, minus-end 2.1 ± 0.5 pN). A diminished peak at 5.2 pN is expected given the reduced number of motors on the lipid droplets in heterozygous embryos compared to wild type.
Figure 5. Droplet motion in wild-type and Khc27/+ embryos is very similar
(A, B, C, D) Distribution of lipid droplet run-lengths in wild-type (A, B) and Khc27/+ (C, D) embryos. Using established approaches (see Experimental Procedures), we employed these distributions to quantify parameters of the two travel states droplets exhibit (Gross et al., 2000): D1 = average travel distance of the short-slow state; D2 = average travel distance of the long-fast state; Davg = mean run length. For both directions of motion, the heterozygotes showed a slight but statistically significant increase in the mean run length (p = 4.8×10-3 for plus-end run lengths; p = 7.27×10-7 for minus-end run lengths; we used a two-sided rank-sum test because of data skewness). (E) Mean travel velocities of lipid droplets for plus-end and minus-end motion. For both directions of motion, Khc27/+ shows a slight but statistically significant increase in the mean velocity of long-fast travel (p = 6.85 ×10-3 for plus-end velocities; p = 3.48 ×10-2 for minus-end velocities; one-sided t-test). Only runs longer than 500 nm in length were used to calculate average velocities. Error bars are standard errors of the mean. (F) Khc27/+ embryos display normal directionality of net droplet transport. Despite reduced Khc protein levels, these embryos display clearing in Phase II and clouding in Phase III, similar to the wild type (Fig. 1A; see also movie S7). (G) Khc levels on droplets remain constant during embryogenesis. Lipid droplets were purified from wild-type embryos of the indicated Phases and analyzed for Khc as in Fig. 4A.
Comment in
- Counting motors by force.
St Johnston D. St Johnston D. Cell. 2008 Dec 12;135(6):1000-1. doi: 10.1016/j.cell.2008.11.021. Cell. 2008. PMID: 19070567
Similar articles
- Counting motors by force.
St Johnston D. St Johnston D. Cell. 2008 Dec 12;135(6):1000-1. doi: 10.1016/j.cell.2008.11.021. Cell. 2008. PMID: 19070567 - Endogenous GSK-3/shaggy regulates bidirectional axonal transport of the amyloid precursor protein.
Weaver C, Leidel C, Szpankowski L, Farley NM, Shubeita GT, Goldstein LS. Weaver C, et al. Traffic. 2013 Mar;14(3):295-308. doi: 10.1111/tra.12037. Epub 2013 Jan 20. Traffic. 2013. PMID: 23279138 - Two kinesins transport cargo primarily via the action of one motor: implications for intracellular transport.
Jamison DK, Driver JW, Rogers AR, Constantinou PE, Diehl MR. Jamison DK, et al. Biophys J. 2010 Nov 3;99(9):2967-77. doi: 10.1016/j.bpj.2010.08.025. Biophys J. 2010. PMID: 21044594 Free PMC article. - Kinesin superfamily motor proteins and intracellular transport.
Hirokawa N, Noda Y, Tanaka Y, Niwa S. Hirokawa N, et al. Nat Rev Mol Cell Biol. 2009 Oct;10(10):682-96. doi: 10.1038/nrm2774. Nat Rev Mol Cell Biol. 2009. PMID: 19773780 Review. - As the fat flies: The dynamic lipid droplets of Drosophila embryos.
Welte MA. Welte MA. Biochim Biophys Acta. 2015 Sep;1851(9):1156-85. doi: 10.1016/j.bbalip.2015.04.002. Epub 2015 Apr 13. Biochim Biophys Acta. 2015. PMID: 25882628 Free PMC article. Review.
Cited by
- Rotational dynamics of cargos at pauses during axonal transport.
Gu Y, Sun W, Wang G, Jeftinija K, Jeftinija S, Fang N. Gu Y, et al. Nat Commun. 2012;3:1030. doi: 10.1038/ncomms2037. Nat Commun. 2012. PMID: 22929787 - Tuning ensemble-averaged cargo run length via fractional change in mean kinesin number.
Wilson JO, Zaragoza AD, Xu J. Wilson JO, et al. Phys Biol. 2021 May 19;18(4):10.1088/1478-3975/abf5b3. doi: 10.1088/1478-3975/abf5b3. Phys Biol. 2021. PMID: 33827070 Free PMC article. - Measuring molecular motor forces in vivo: implications for tug-of-war models of bidirectional transport.
Leidel C, Longoria RA, Gutierrez FM, Shubeita GT. Leidel C, et al. Biophys J. 2012 Aug 8;103(3):492-500. doi: 10.1016/j.bpj.2012.06.038. Biophys J. 2012. PMID: 22947865 Free PMC article. - Coordination of molecular motors: from in vitro assays to intracellular dynamics.
Holzbaur EL, Goldman YE. Holzbaur EL, et al. Curr Opin Cell Biol. 2010 Feb;22(1):4-13. doi: 10.1016/j.ceb.2009.12.014. Epub 2010 Jan 25. Curr Opin Cell Biol. 2010. PMID: 20102789 Free PMC article. - Single-molecule fluorescence and in vivo optical traps: how multiple dyneins and kinesins interact.
Blehm BH, Selvin PR. Blehm BH, et al. Chem Rev. 2014 Mar 26;114(6):3335-52. doi: 10.1021/cr4005555. Epub 2014 Jan 23. Chem Rev. 2014. PMID: 24666199 Free PMC article. Review. No abstract available.
References
- Carter BC, Shubeita GT, Gross SP. Tracking single particles: a user-friendly quantitative evaluation. Phys Biol. 2005;2:60–72. - PubMed
- Carter NJ, Cross RA. Mechanics of the kinesin step. Nature. 2005;435:308–312. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GM64687/GM/NIGMS NIH HHS/United States
- R01 GM064687-07/GM/NIGMS NIH HHS/United States
- S10 RR024577/RR/NCRR NIH HHS/United States
- R01 GM064624/GM/NIGMS NIH HHS/United States
- GM64624/GM/NIGMS NIH HHS/United States
- R01 GM064624-05/GM/NIGMS NIH HHS/United States
- T32 GM007122/GM/NIGMS NIH HHS/United States
- R01 GM064687/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous