Concurrent regulation of AMP-activated protein kinase and SIRT1 in mammalian cells - PubMed (original) (raw)
Concurrent regulation of AMP-activated protein kinase and SIRT1 in mammalian cells
Gabriela Suchankova et al. Biochem Biophys Res Commun. 2009.
Abstract
We examined in HepG2 cells whether glucose-induced changes in AMP-activated protein kinase (AMPK) activity could be mediated by SIRT1, an NAD(+)-dependent histone/protein deacetylase that has been linked to the increase in longevity caused by caloric restriction. Incubation with 25 vs. 5mM glucose for 6h concurrently diminished the phosphorylation of AMPK (Thr 172) and ACC (Ser 79), increased lactate release, and decreased the abundance and activity of SIRT1. In contrast, incubation with pyruvate (0.1 and 1mM) for 2h increased AMPK phosphorylation and SIRT1 abundance and activity. The putative SIRT1 activators resveratrol and quercetin also increased AMPK phosphorylation. None of the tested compounds (low or high glucose, pyruvate, and resveratrol) significantly altered the AMP/ATP ratio. Collectively, these findings raise the possibility that glucose-induced changes in AMPK are linked to alterations in SIRT1 abundance and activity and possibly cellular redox state.
Figures
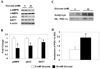
Figure 1. Increasing the ambient glucose concentration decreases the phosphorylation of AMPK and ACC, and the abundance and activity of SIRT1 in HepG2 cells
HepG2 cells incubated in 5 or 25 mM glucose for 6 h. (A) Western blot analysis for p-AMPK (Thr 172), p-ACC (Ser 79), and SIRT1. (B) Quantification of representative blot shown in A, results are means ± SE (n=5); *,p<0.05. (C) PGC-1α acetylation after 6 h incubation in 5 or 25 mM glucose. (D) Quantification of Acetyl-Lys/HA-PGC-1α. Results are means ± SE, (n = 4); *, p<0.05.
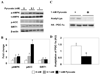
Figure 2. Increasing pyruvate concentration increases p-AMPK, p-ACC, and SIRT1 abundance and activity
(A) Western blot analysis of HepG2 cells following 2 h incubation with the indicated concentrations of pyruvate. (B) Quantification of p-AMPK, p-ACC, and SIRT1 relative to β-actin. Results are means ± SE (n = 6); *, p<0.05 vs. 0 mM. (C) PGC-1α acetylation after 2 h incubation ± 1 mM pyruvate. (D) Quantification of Acetyl-Lys/HA-PGC-1α. Results are means ± SE, (n = 4); *, p<0.05.
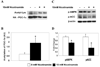
Figure 3. Nicotinamide increases PGC-1α acetylation and decreases the phosphorylation of AMPK and ACC
(A) PGC-1α acetylation in HepG2 cells incubated ± 10 mM nicotinamide (NAM) for 6 h. (B) Quantification of Acetyl-Lys/HA-PGC-1α. Results are means ± SE, (n = 4); *, p<0.05. (C) Western blot analysis of p-AMPK and p-ACC. (D) Quantification of representative blot shown in C. Results are means ± SE, (n = 4); *, p<0.05.
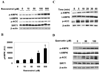
Figure 4. Resveratrol and quercetin increase the phosphorylation of AMPK and ACC in HepG2 cells
(A, B) Dose response of p-AMPK and p-ACC to 1 h resveratrol incubation. Results are means ± SE (n = 2); *,p<0.05 vs. 0 µM resveratrol. (C) Time course of changes in p-AMPK and p-ACC caused by 100 µM resveratrol. (D) Western blot analysis showing enhanced p-AMPK and p-ACC following 1 h incubation with increasing concentrations of quercetin.
Similar articles
- Resveratrol-Induced AMP-Activated Protein Kinase Activation Is Cell-Type Dependent: Lessons from Basic Research for Clinical Application.
Lan F, Weikel KA, Cacicedo JM, Ido Y. Lan F, et al. Nutrients. 2017 Jul 14;9(7):751. doi: 10.3390/nu9070751. Nutrients. 2017. PMID: 28708087 Free PMC article. - Role of deleted in breast cancer 1 (DBC1) protein in SIRT1 deacetylase activation induced by protein kinase A and AMP-activated protein kinase.
Nin V, Escande C, Chini CC, Giri S, Camacho-Pereira J, Matalonga J, Lou Z, Chini EN. Nin V, et al. J Biol Chem. 2012 Jul 6;287(28):23489-501. doi: 10.1074/jbc.M112.365874. Epub 2012 May 2. J Biol Chem. 2012. PMID: 22553202 Free PMC article. - SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase.
Hou X, Xu S, Maitland-Toolan KA, Sato K, Jiang B, Ido Y, Lan F, Walsh K, Wierzbicki M, Verbeuren TJ, Cohen RA, Zang M. Hou X, et al. J Biol Chem. 2008 Jul 18;283(29):20015-26. doi: 10.1074/jbc.M802187200. Epub 2008 May 14. J Biol Chem. 2008. PMID: 18482975 Free PMC article. - Sensing of energy and nutrients by AMP-activated protein kinase.
Hardie DG. Hardie DG. Am J Clin Nutr. 2011 Apr;93(4):891S-6. doi: 10.3945/ajcn.110.001925. Epub 2011 Feb 16. Am J Clin Nutr. 2011. PMID: 21325438 Review. - AMP-activated protein kinase: also regulated by ADP?
Hardie DG, Carling D, Gamblin SJ. Hardie DG, et al. Trends Biochem Sci. 2011 Sep;36(9):470-7. doi: 10.1016/j.tibs.2011.06.004. Epub 2011 Jul 23. Trends Biochem Sci. 2011. PMID: 21782450 Review.
Cited by
- Sirtuin 1 promotes the growth and cisplatin resistance of endometrial carcinoma cells: a novel therapeutic target.
Asaka R, Miyamoto T, Yamada Y, Ando H, Mvunta DH, Kobara H, Shiozawa T. Asaka R, et al. Lab Invest. 2015 Dec;95(12):1363-73. doi: 10.1038/labinvest.2015.119. Epub 2015 Sep 14. Lab Invest. 2015. PMID: 26367491 - Regulation of yak longissimus lumborum energy metabolism and tenderness by the AMPK/SIRT1 signaling pathways during postmortem storage.
Yang Y, Yang J, Yu Q, Gao Y, Zheng Y, Han L, Ding X. Yang Y, et al. PLoS One. 2022 Nov 28;17(11):e0277410. doi: 10.1371/journal.pone.0277410. eCollection 2022. PLoS One. 2022. PMID: 36441689 Free PMC article. - Downregulation of AMPK accompanies leucine- and glucose-induced increases in protein synthesis and insulin resistance in rat skeletal muscle.
Saha AK, Xu XJ, Lawson E, Deoliveira R, Brandon AE, Kraegen EW, Ruderman NB. Saha AK, et al. Diabetes. 2010 Oct;59(10):2426-34. doi: 10.2337/db09-1870. Epub 2010 Aug 3. Diabetes. 2010. PMID: 20682696 Free PMC article. - AMPK and SIRT1: a long-standing partnership?
Ruderman NB, Xu XJ, Nelson L, Cacicedo JM, Saha AK, Lan F, Ido Y. Ruderman NB, et al. Am J Physiol Endocrinol Metab. 2010 Apr;298(4):E751-60. doi: 10.1152/ajpendo.00745.2009. Epub 2010 Jan 26. Am J Physiol Endocrinol Metab. 2010. PMID: 20103737 Free PMC article. Review. - Interplay between Systemic Glycemia and Neuroprotective Activity of Resveratrol in Modulating Astrocyte SIRT1 Response to Neuroinflammation.
Grabowska AD, Wątroba M, Witkowska J, Mikulska A, Sepúlveda N, Szukiewicz D. Grabowska AD, et al. Int J Mol Sci. 2023 Jul 19;24(14):11640. doi: 10.3390/ijms241411640. Int J Mol Sci. 2023. PMID: 37511397 Free PMC article.
References
- Hardie DG, Scott JW, Pan DA, Hudson ER. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett. 2003;546:113–120. - PubMed
- Kahn B, Alquier T, Carling D, Hardie G. AMP-activated protein kinase: Ancient energy gauge provides clues to modern understanding of metabolism. Cell Metabolism. 2005;1:15–25. - PubMed
- Zang M, Zuccollo A, Hou X, Nagata D, Walsh K, Herscovitz H, Brecher P, Ruderman NB, Cohen RA. AMP-activated protein kinase is required for the lipid-lowering effect of metformin in insulin-resistant human HepG2 cells. J Biol Chem. 2004;279:47898–47905. - PubMed
- Kemp BE, Stapleton D, Campbell DJ, Chen ZP, Murthy S, Walter M, Gupta A, Adams JJ, Katsis F, van Denderen B, Jennings IG, Iseli T, Michell BJ, Witters LA. AMP-activated protein kinase, super metabolic regulator. Biochem Soc Trans. 2003;31:162–168. - PubMed
- Beauloye C, Marsin AS, Bertrand L, Krause U, Hardie DG, Vanoverschelde JL, Hue L. Insulin antagonizes AMP-activated protein kinase activation by ischemia or anoxia in rat hearts, without affecting total adenine nucleotides. FEBS Lett. 2001;505:348–352. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK067509/DK/NIDDK NIH HHS/United States
- F30 DK082136/DK/NIDDK NIH HHS/United States
- T32 DK007201/DK/NIDDK NIH HHS/United States
- R01 DK19514/DK/NIDDK NIH HHS/United States
- P01 HL08758/HL/NHLBI NIH HHS/United States
- T32DK07201-31/DK/NIDDK NIH HHS/United States
- DK67509/DK/NIDDK NIH HHS/United States
- F30DK082136/DK/NIDDK NIH HHS/United States
- P01 HL068758/HL/NHLBI NIH HHS/United States
- R01 DK019514/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources