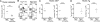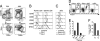Dendritic cells in the thymus contribute to T-regulatory cell induction - PubMed (original) (raw)
. 2008 Dec 16;105(50):19869-74.
doi: 10.1073/pnas.0810268105. Epub 2008 Dec 10.
Serani van Dommelen, Penghui Zhou, Alexandra Rizzitelli, Angela D'Amico, Raymond J Steptoe, Shalin H Naik, Mireille H Lahoud, Yang Liu, Pan Zheng, Ken Shortman, Li Wu
Affiliations
- PMID: 19073916
- PMCID: PMC2604962
- DOI: 10.1073/pnas.0810268105
Dendritic cells in the thymus contribute to T-regulatory cell induction
Anna I Proietto et al. Proc Natl Acad Sci U S A. 2008.
Erratum in
- Proc Natl Acad Sci U S A. 2009 Feb 3;106(5):1679
Abstract
Central tolerance is established through negative selection of self-reactive thymocytes and the induction of T-regulatory cells (T(R)s). The role of thymic dendritic cells (TDCs) in these processes has not been clearly determined. In this study, we demonstrate that in vivo, TDCs not only play a role in negative selection but in the induction of T(R)s. TDCs include two conventional dendritic cell (DC) subtypes, CD8(lo)Sirpalpha(hi/+) (CD8(lo)Sirpalpha(+)) and CD8(hi)Sirpalpha(lo/-) (CD8(hi)Sirpalpha(-)) [corrected] which have different origins. We found that the CD8(hi)Sirpalpha(+) DCs represent a conventional DC subset that originates from the blood and migrates into the thymus. Moreover, we show that the CD8(lo)Sirpalpha(+) DCs demonstrate a superior capacity to induce T(R)s in vitro. Finally, using a thymic transplantation system, we demonstrate that the DCs in the periphery can migrate into the thymus, where they efficiently induce T(R) generation and negative selection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
MHCII+ DCs contribute to negative selection and TR induction. (A) The light density fraction of cells from the thymus of WT and MHCII−/− BM chimeras was analyzed to determine the % of donor-derived DCs. More than 98% of CD11c+ cells were CD45.2+ donor-derived DCs in both BM chimera groups. (B) MHCII expression on CD11c+ TDCs in WT (black line) and MHCII−/− (gray line) chimeras. (C) The % of double-negative, double-positive, CD4+, and CD8+ thymocytes (Upper) and CD4+CD25+Foxp3+ TRs (Lower). (D) The % and total number of CD4+ thymocytes in WT and MHCII−/− BM chimeras. (E) The % and total number of TRs in WT and MHCII−/− BM chimeras (n = 20–24 per group for D and E). (F) Suppressive activity of CD4+CD25+ thymocytes from MHCII−/− or WT chimeras. Data are the mean (error bars, ±SD) of triplicate cultures from one of two experiments. (G) The number of TRs (CD4+Foxp3+) in the thymus of B6 to B6, B6 to B7−/−, B7−/− to B6, and B7−/− to B7−/− BM chimeras was analyzed by flow cytometry. Data are the mean of three independent experiments (error bars, ±SD) (n = 68 for G). *, P < 0.05; **, P < 0.001; ***, P < 0.0001.
Fig. 2.
OVA expressing DCs induce OTII TRs and deletion of OTII CD4+ T cells. Irradiated WT CD45.1 mice were reconstituted with double-tg Rag2−/−OTII/CD11cOVA (Rag2−/−O/OVA) BM or Rag2−/−OTII BM as a control (n = 4–5 per group). (A) Total thymic cellularity of Rag2−/−O/OVA and control BM chimeras. (B) CD45.2+Vα2+ thymocytes were gated, and the % of CD4+ and CD8+ thymocytes was determined. To assess TR induction, CD4+ OTII+ thymocytes were gated for and expression of CD25 and Foxp3 was determined. The % and number of OTII+ CD4+ thymocytes (C) and the % and number of TRs (D) in Rag2−/−O/OVA and control BM chimeras.
Fig. 3.
Sirpα+ TcDCs are more mature and efficiently induce TRs in vitro. (A) Enriched TDCs and SDCs were segregated into pDCs (CD11cintCD45RA+) and cDCs (CD11c+CD45RA−) and further segregated into CD8loSirpα+ and CD8hiSirp− cDCs. Thymic and splenic Sirpα+ and Sirpα− cDCs (B) and thymic and splenic pDCs (C) were analyzed for the expression of costimulatory molecules, CD69, and MHCII. (D) TR (CD4+CD25+Foxp3+) induction by TDC subsets in the cocultures of TDCs and CD4+CD25− thymocytes for 5 days. Data shown are representative of six experiments. (E) The number of TRs induced was determined by flow cytometric analysis of the cultured cells of D using calibration beads (n = 6) (error bars, ±SD). (F) Suppressive capacity of TRs induced in cultures by Sirpα+ TcDCs. In vitro_–derived TRs were sorted as CD4+CD25+CD62L+ and subject to a suppression assay as per Fig. 1_F. The number of proliferating CFSE-labeled CD4+CD25− T cells was determined 3 days later (n = 2).
Fig. 4.
Sirpα+ TcDCs originate from peripheral blood and can migrate into the thymus. (A) DC generation from purified Lineage−Thy-1loc-kit+ intrathymic precursors (CD45.2) was analyzed 2 weeks after precursor transfer. The intrathymic precursor-derived cDCs were mainly CD8+Sirpα− (Right). A representative contour plot of the normal TcDC subsets is shown (Left) for comparison. (B) White blood cells (20 × 106) from CD45.1 mice were transferred i.v. into nonirradiated CD45.2 recipients. The phenotype of donor-derived cells in the thymus of recipients was determined 3 days later by gating for CD45.1+CD11c+CD45RAlo cDCs. Expression of Sirpα, CD11b, CD8, and MHCII was determined on this population. (C–E) Thymic lobes from OTII tg CD45.2+ mice crossed to CD45.1+ WT mice were grafted under the kidney capsule of CD45.2+ CD11cOVA tg or WT recipients. (C) The phenotype of recipient-derived CD45.2+CD45.1− DCs in the grafted thymic lobes from WT and CD11cOVA tg mice was determined. The recipient CD45.2+CD45.1−CD11c+CD45RA− cDCs were gated for, and the expression of CD8 and Sirpα was determined. The level of expression of MHCII was determined on Sirpα− and Sirpα+ cDCs. (D) The total number of CD45.1+CD4+Vα2+Vβ5+ cells (OTII) was calculated in OTII lobes grafted into WT or CD11cOVA tg recipients. Data are the mean of three independent experiments (error bars, ±SD) (n = 11–21). *, P < 0.05. (E) CD45.1+CD4+Vα2+Vβ5+ cells in the OTII lobes from WT and CD11cOVA tg recipients (as in D) were further analyzed for CD25 and Foxp3 expression. The total number of CD45.1+CD4+Vα2+Vβ5+CD25+Foxp3+ TRs was calculated. Data are the mean of three independent experiments (error bars, ±SD) (n = 11–21). *, P < 0.05.
Similar articles
- Medullary thymic epithelial cells and CD8α+ dendritic cells coordinately regulate central tolerance but CD8α+ cells are dispensable for thymic regulatory T cell production.
Herbin O, Bonito AJ, Jeong S, Weinstein EG, Rahman AH, Xiong H, Merad M, Alexandropoulos K. Herbin O, et al. J Autoimmun. 2016 Dec;75:141-149. doi: 10.1016/j.jaut.2016.08.002. Epub 2016 Aug 16. J Autoimmun. 2016. PMID: 27543048 Free PMC article. - The impact of circulating dendritic cells on the development and differentiation of thymocytes.
Proietto AI, van Dommelen S, Wu L. Proietto AI, et al. Immunol Cell Biol. 2009 Jan;87(1):39-45. doi: 10.1038/icb.2008.86. Epub 2008 Dec 2. Immunol Cell Biol. 2009. PMID: 19048018 Review. - CCR7 Modulates the Generation of Thymic Regulatory T Cells by Altering the Composition of the Thymic Dendritic Cell Compartment.
Hu Z, Li Y, Van Nieuwenhuijze A, Selden HJ, Jarrett AM, Sorace AG, Yankeelov TE, Liston A, Ehrlich LIR. Hu Z, et al. Cell Rep. 2017 Oct 3;21(1):168-180. doi: 10.1016/j.celrep.2017.09.016. Cell Rep. 2017. PMID: 28978470 Free PMC article. - Crucial contribution of thymic Sirp alpha+ conventional dendritic cells to central tolerance against blood-borne antigens in a CCR2-dependent manner.
Baba T, Nakamoto Y, Mukaida N. Baba T, et al. J Immunol. 2009 Sep 1;183(5):3053-63. doi: 10.4049/jimmunol.0900438. Epub 2009 Aug 12. J Immunol. 2009. PMID: 19675159 - Heterogeneity of thymic dendritic cells.
Wu L, Shortman K. Wu L, et al. Semin Immunol. 2005 Aug;17(4):304-12. doi: 10.1016/j.smim.2005.05.001. Semin Immunol. 2005. PMID: 15946853 Review.
Cited by
- Conserved and divergent aspects of human T-cell development and migration in humanized mice.
Halkias J, Yen B, Taylor KT, Reinhartz O, Winoto A, Robey EA, Melichar HJ. Halkias J, et al. Immunol Cell Biol. 2015 Sep;93(8):716-26. doi: 10.1038/icb.2015.38. Epub 2015 Mar 6. Immunol Cell Biol. 2015. PMID: 25744551 Free PMC article. - T-cell tolerance: central and peripheral.
Xing Y, Hogquist KA. Xing Y, et al. Cold Spring Harb Perspect Biol. 2012 Jun 1;4(6):a006957. doi: 10.1101/cshperspect.a006957. Cold Spring Harb Perspect Biol. 2012. PMID: 22661634 Free PMC article. - Murine myeloproliferative disorder as a consequence of impaired collaboration between dendritic cells and CD4 T cells.
Humblet-Baron S, Barber JS, Roca CP, Lenaerts A, Koni PA, Liston A. Humblet-Baron S, et al. Blood. 2019 Jan 24;133(4):319-330. doi: 10.1182/blood-2018-05-850321. Epub 2018 Oct 17. Blood. 2019. PMID: 30333120 Free PMC article. - Self-antigen presentation by dendritic cells in autoimmunity.
Hopp AK, Rupp A, Lukacs-Kornek V. Hopp AK, et al. Front Immunol. 2014 Feb 13;5:55. doi: 10.3389/fimmu.2014.00055. eCollection 2014. Front Immunol. 2014. PMID: 24592266 Free PMC article. Review. - Medullary thymic epithelial cells and CD8α+ dendritic cells coordinately regulate central tolerance but CD8α+ cells are dispensable for thymic regulatory T cell production.
Herbin O, Bonito AJ, Jeong S, Weinstein EG, Rahman AH, Xiong H, Merad M, Alexandropoulos K. Herbin O, et al. J Autoimmun. 2016 Dec;75:141-149. doi: 10.1016/j.jaut.2016.08.002. Epub 2016 Aug 16. J Autoimmun. 2016. PMID: 27543048 Free PMC article.
References
- Palmer E. Negative selection—clearing out the bad apples from the T-cell repertoire. Nat Rev Immunol. 2003;3:383–391. - PubMed
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. - PubMed
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science (New York, NY) 2003;299:1057–1061. - PubMed
- Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. - PubMed
- Jordan MS, et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–306. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials