Growth-rate-dependent partitioning of RNA polymerases in bacteria - PubMed (original) (raw)
Growth-rate-dependent partitioning of RNA polymerases in bacteria
Stefan Klumpp et al. Proc Natl Acad Sci U S A. 2008.
Abstract
Physiological changes that result in changes in bacterial gene expression are often accompanied by changes in the growth rate for fast adapting enteric bacteria. Because the availability of RNA polymerase (RNAP) in cells depends on the growth rate, transcriptional control involves not only the regulation of promoters, but also depends on the available (or free) RNAP concentration, which is difficult to quantify directly. Here, we develop a simple physical model describing the partitioning of cellular RNAP into different classes: RNAPs transcribing mRNA and ribosomal RNA (rRNA), RNAPs nonspecifically bound to DNA, free RNAP, and immature RNAP. Available experimental data for Escherichia coli allow us to determine the 2 unknown parameters of the model and hence deduce the free RNAP concentration at different growth rates. The results allow us to predict the growth-rate dependence of the activities of constitutive (unregulated) promoters, and to disentangle the growth-rate-dependent regulation of promoters (e.g., the promoters of rRNA operons) from changes in transcription due to changes in the free RNAP concentration at different growth rates. Our model can quantitatively account for the observed changes in gene expression patterns in mutant E. coli strains with altered levels of RNAP expression without invoking additional parameters. Applying our model to the case of the stringent response after amino acid starvation, we can evaluate the plausibility of various scenarios of passive transcriptional control proposed to account for the observed changes in the expression of rRNA and biosynthetic operons.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Model for the partitioning of RNAPs. In exponentially growing cells all RNAPs are taken to fall into 5 classes, RNAPs transcribing mRNA (_N_m) and rRNA (_N_r), RNAPs nonspecifically bound to DNA (_N_ns), free RNAPs (_N_free), and RNAP assembly intermediates (immature RNAPs, _N_interm). The total number of RNAPs per cell (_N_RNAP) is the sum of the number of RNAPs in these classes. Our model describes the numbers of RNAPs in each class by equations that link them to measured biophysical parameters of the cell (see
SI Text
for a detailed description and
Tables S1 and S2
for the parameter values, many of which are growth-rate dependent). The numbers of transcribing RNAPs (_N_r and _N_m) are both described by a microscopic model Eqs. 1a, 2a, and estimated directly from measured RNA synthesis rates Eqs. 1b and 2b.
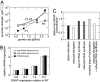
Fig. 2.
Partitioning of RNAPs at different growth rates. (A) Total number of RNAPs per cell and numbers of RNAPs in the different classes as predicted by our model. (B) Concentrations of total RNAP and RNAPs in the different classes.
Fig. 3.
Growth-rate-dependent transcription from constitutive promoters. Growth-rate dependence of the transcription rates from several constitutive promoters and the rrn promoter P2. Data are taken from ref. and have been normalized to the maximal value per promoter. (For P2 we also included corresponding data from ref. .) The black curve indicates the free RNAP concentration from Fig. 2, which is proportional to the predicted transcription rate from an unsaturated constitutive promoter.
Fig. 4.
Consequences of the predicted free RNAP concentration. (A) Growth-rate-dependent regulation of the rrn promoters: Effective promoter strengths for the rrn promoters P1 (black), P2 (gray), and the pair P1-P2 (white) as calculated from the transcription rates measured in ref. . (B) Predicted mRNA expression for over- and under expression of RNAP and comparison with data from ref. . (C) Passive control during the stringent response: Concentration of free RNAPs during the stringent response relative to the concentration during the exponential growth (with a rate of 2.5 doublings per hour) before starvation.
Similar articles
- Regulation of rRNA transcription is remarkably robust: FIS compensates for altered nucleoside triphosphate sensing by mutant RNA polymerases at Escherichia coli rrn P1 promoters.
Bartlett MS, Gaal T, Ross W, Gourse RL. Bartlett MS, et al. J Bacteriol. 2000 Apr;182(7):1969-77. doi: 10.1128/JB.182.7.1969-1977.2000. J Bacteriol. 2000. PMID: 10715005 Free PMC article. - RNA polymerase redistribution supports growth in E. coli strains with a minimal number of rRNA operons.
Fan J, El Sayyed H, Pambos OJ, Stracy M, Kyropoulos J, Kapanidis AN. Fan J, et al. Nucleic Acids Res. 2023 Aug 25;51(15):8085-8101. doi: 10.1093/nar/gkad511. Nucleic Acids Res. 2023. PMID: 37351576 Free PMC article. - Growth rate regulation in Escherichia coli.
Jin DJ, Cagliero C, Zhou YN. Jin DJ, et al. FEMS Microbiol Rev. 2012 Mar;36(2):269-87. doi: 10.1111/j.1574-6976.2011.00279.x. Epub 2011 Jun 3. FEMS Microbiol Rev. 2012. PMID: 21569058 Free PMC article. Review. - Transcription factor dynamics.
Lewis PJ, Doherty GP, Clarke J. Lewis PJ, et al. Microbiology (Reading). 2008 Jul;154(Pt 7):1837-1844. doi: 10.1099/mic.0.2008/018549-0. Microbiology (Reading). 2008. PMID: 18599813 Review.
Cited by
- Isocost Lines Describe the Cellular Economy of Genetic Circuits.
Gyorgy A, Jiménez JI, Yazbek J, Huang HH, Chung H, Weiss R, Del Vecchio D. Gyorgy A, et al. Biophys J. 2015 Aug 4;109(3):639-46. doi: 10.1016/j.bpj.2015.06.034. Biophys J. 2015. PMID: 26244745 Free PMC article. - Emergent expression of fitness-conferring genes by phenotypic selection.
Ciechonska M, Sturrock M, Grob A, Larrouy-Maumus G, Shahrezaei V, Isalan M. Ciechonska M, et al. PNAS Nexus. 2022 Jun 10;1(3):pgac069. doi: 10.1093/pnasnexus/pgac069. eCollection 2022 Jul. PNAS Nexus. 2022. PMID: 36741458 Free PMC article. - Spatial distribution and diffusive motion of RNA polymerase in live Escherichia coli.
Bratton BP, Mooney RA, Weisshaar JC. Bratton BP, et al. J Bacteriol. 2011 Oct;193(19):5138-46. doi: 10.1128/JB.00198-11. Epub 2011 Jul 22. J Bacteriol. 2011. PMID: 21784927 Free PMC article. - Dynamical model of antibiotic responses linking expression of resistance genes to metabolism explains emergence of heterogeneity during drug exposures.
Stevanovic M, Teuber Carvalho JP, Bittihn P, Schultz D. Stevanovic M, et al. Phys Biol. 2024 Apr 2;21(3):036002. doi: 10.1088/1478-3975/ad2d64. Phys Biol. 2024. PMID: 38412523 Free PMC article. - Lag phase is a distinct growth phase that prepares bacteria for exponential growth and involves transient metal accumulation.
Rolfe MD, Rice CJ, Lucchini S, Pin C, Thompson A, Cameron AD, Alston M, Stringer MF, Betts RP, Baranyi J, Peck MW, Hinton JC. Rolfe MD, et al. J Bacteriol. 2012 Feb;194(3):686-701. doi: 10.1128/JB.06112-11. Epub 2011 Dec 2. J Bacteriol. 2012. PMID: 22139505 Free PMC article.
References
- Schaechter M, Maaløe O, Kjeldgaard NO. Dependency on medium and temperature of cell size and chemical composition during balanced growth of Salmonella typhimurium. J Gen Microbiol. 1958;19:592–606. - PubMed
- Neidhardt FC, Ingraham JL, Schaechter M. Physiology of the Bacterial Cell: A Molecular Approach. Sunderland, MA: Sinauer; 1990.
- Maaloe O. An analysis of bacterial growth. Dev Biol Suppl. 1969;3:33–58.
- Bremer H, Dennis PP. Modulation of chemical composition and other parameters of the cell by growth rate. In: Neidhardt FC, editor. Escherichia coli and Salmonella. 2nd Ed. Washington DC: ASM Press; 1996. pp. 1553–1569.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources